Integrated Stress Response on:
[Wikipedia]
[Google]
[Amazon]
The integrated stress response is a
 The integrated stress response can be triggered within a cell due to either extrinsic or intrinsic conditions. Extrinsic factors include hypoxia,
The integrated stress response can be triggered within a cell due to either extrinsic or intrinsic conditions. Extrinsic factors include hypoxia,
 PERK (encoded in humans by the gene ''EIF2AK3'') responds mainly to
PERK (encoded in humans by the gene ''EIF2AK3'') responds mainly to 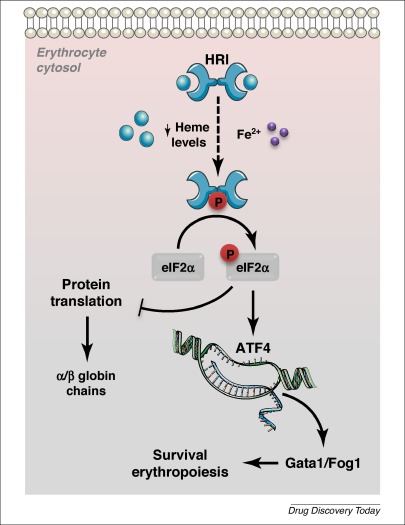

cellular stress response Cellular stress response is the wide range of molecular changes that cells undergo in response to environmental stressors, including extremes of temperature, exposure to toxins, and mechanical damage. Cellular stress responses can also be caused b ...
conserved in eukaryotic
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
cells that downregulates protein synthesis
Protein biosynthesis (or protein synthesis) is a core biological process, occurring inside Cell (biology), cells, homeostasis, balancing the loss of cellular proteins (via Proteolysis, degradation or Protein targeting, export) through the product ...
and upregulates specific genes in response to internal or environmental stresses.
Background
 The integrated stress response can be triggered within a cell due to either extrinsic or intrinsic conditions. Extrinsic factors include hypoxia,
The integrated stress response can be triggered within a cell due to either extrinsic or intrinsic conditions. Extrinsic factors include hypoxia, amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
deprivation, glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
deprivation, viral infection
A viral disease (or viral infection) occurs when an organism's body is invaded by pathogenic viruses, and infectious virus particles (virions) attach to and enter susceptible cells.
Structural Characteristics
Basic structural characteristics, s ...
and presence of oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
s. The main intrinsic factor is endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
stress due to the accumulation of unfolded proteins. It has also been observed that the integrated stress response may trigger due to oncogene
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.
activation. The integrated stress response will either cause the expression of genes
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. Th ...
that fix the damage in the cell due to the stressful conditions, or it will cause a cascade of events leading to apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
, which occurs when the cell cannot be brought back into homeostasis
In biology, homeostasis (British English, British also homoeostasis) Help:IPA/English, (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physics, physical, and chemistry, chemical conditions maintained by organism, living systems. Thi ...
.
eIF2 protein complex
Stress signals can causeprotein kinase
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a fu ...
s, known as EIF-2 kinases, to phosphorylate the α subunit of a protein complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multienzyme complexes, in which multiple catalytic domains are found in a single polypeptide chain.
Protein c ...
called translation initiation factor 2 (eIF2), resulting in the gene ATF4
Activating transcription factor 4 (tax-responsive enhancer element B67), also known as ATF4, is a protein that in humans is encoded by the ''ATF4'' gene.
Function
This gene encodes a transcription factor that was originally identified as a wi ...
being turned on, which will further affect gene expression. eIF2 consists of three subunits: eIF2α, eIF2β and eIF2γ. eIF2α contains two binding sites, one for phosphorylation and one for RNA binding. The kinases work to phosphorylate serine 51 on the α subunit, which is a reversible action. In a cell experiencing normal conditions, eIF2 aids in the initiation of mRNA translation and recognizing the AUG start codon. However, once eIF2α is phosphorylated, the complex’s activity reduces, causing reduction in translation initiation and protein synthesis, while promoting expression of the ATF4 gene.
Protein kinases
There are four knownmammalian
Mammals () are a group of vertebrate animals constituting the class (biology), class Mammalia (), characterized by the presence of mammary glands which in Female#Mammalian female, females produce milk for feeding (nursing) their young, a ...
protein kinase
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a fu ...
s that phosphorylate eIF2α, including PKR-like ER kinase (PERK, EIF2AK3), heme-regulated eIF2α kinase (HRI, EIF2AK1), general control non-depressible 2 (GCN2, EIF2AK4) and double stranded RNA dependent protein kinase (PKR, EIF2AK2).
PERK
 PERK (encoded in humans by the gene ''EIF2AK3'') responds mainly to
PERK (encoded in humans by the gene ''EIF2AK3'') responds mainly to endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
stress and has two modes of activation. This kinase has a unique luminal domain that plays a role in activation. The classical model of activation states that the luminal domain is normally bound to 78-kDa glucose-regulated protein (GRP78
Binding immunoglobulin protein (BiP) also known as 78 kDa glucose-regulated protein (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) is a protein that in humans is encoded by the ''HSPA5'' gene.
BiP is a HSP70 molecular chaperone located in the l ...
). Once there is a buildup of unfolded proteins, GRP78 dissociates from the luminal domain. This causes PERK to dimerize, leading to autophosphorylation and activation. The activated PERK kinase will then phosphorylate eIF2α, causing a cascade of events. Thus, the activation of this kinase is dependent on the aggregation of unfolded proteins in the endoplasmic reticulum. PERK has also been observed to activate in response to activity of the proto-oncogene MYC
''Myc'' is a family of regulator genes and proto-oncogenes that code for transcription factors. The ''Myc'' family consists of three related human genes: ''c-myc'' (MYC), ''l-myc'' ( MYCL), and ''n-myc'' (MYCN). ''c-myc'' (also sometimes refe ...
. This activation causes ATF4 expression, resulting in tumorigenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abno ...
and cellular transformation
In molecular biology and genetics, transformation is the genetic alteration of a cell resulting from the direct uptake and incorporation of exogenous genetic material from its surroundings through the cell membrane(s). For transformation to ta ...
.

HRI
HRI (encoded in humans by the gene ''EIF2AK1'') also dimerizes in order to autophosphorylate and activate. This activation is dependent on the presence ofheme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consisti ...
. HRI has two domains that heme may bind to, including one on the N-terminus and one on the kinase insertion domain. The presence of heme causes a disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
to form between the monomers of HRI, resulting in the structure of an inactive dimer. However, when heme is absent, HRI monomers form an active dimer through non-covalent interactions. Therefore, the activation of this kinase is dependent on heme deficiency. HRI activation can also occur due to other stressors such as heat shock, osmotic stress and proteasome inhibition. Activation of HRI in response to these stressors does not depend on heme, but rather relies on the help of two heat shock proteins (HSP90
Hsp90 (heat shock protein 90) is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, ...
and HSP70
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an importa ...
). HRI is mainly found in the precursors of red blood cells, and has been observed to increase during erythropoiesis
Erythropoiesis (from Greek 'erythro' meaning "red" and 'poiesis' "to make") is the process which produces red blood cells (erythrocytes), which is the development from erythropoietic stem cell to mature red blood cell.
It is stimulated by decrea ...
.

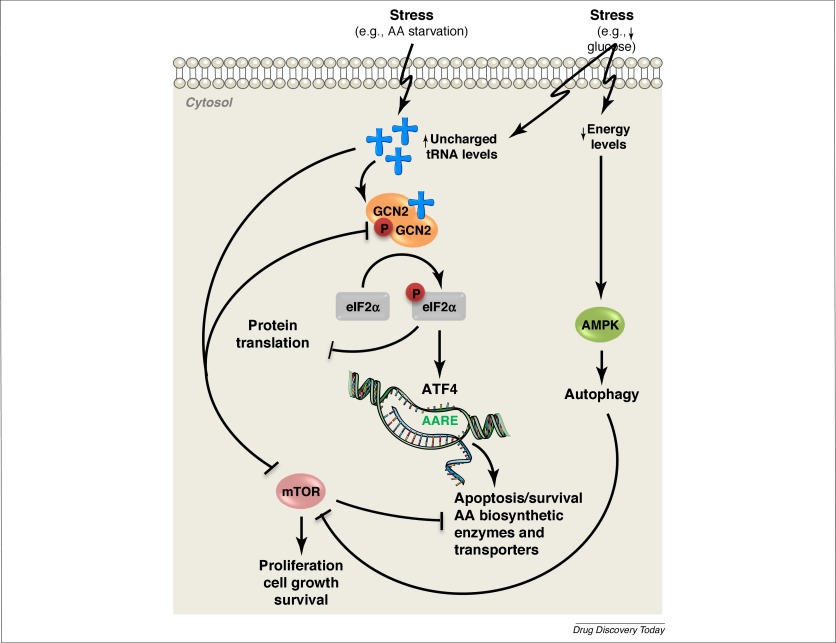
GCN2

GCN2
GCN2 (general control nonderepressible 2) is a serine/threonine-protein kinase that senses amino acid deficiency through binding to uncharged transfer RNA (tRNA). It plays a key role in modulating amino acid metabolism as a response to nutrient d ...
(encoded in humans by the gene ''EIF2AK4'') is activated as a result of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
deprivation. The mechanisms regarding this activation are still being researched; however, one mechanism has been studied in yeast. It was observed that GCN2 binds to uncharged/deacylated tRNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ac ...
which causes a conformational change, resulting in dimerization. Dimerization then causes autophosphorylation and activation. Other stressors have also been reported to activate GCN2. GCN2 activation was observed in glucose deprived tumor cells, although it was suggested that it was an indirect effect due to cells using amino acids as an alternate energy source. In mouse embryonic fibroblast
A fibroblast is a type of cell (biology), biological cell that synthesizes the extracellular matrix and collagen, produces the structural framework (Stroma (tissue), stroma) for animal Tissue (biology), tissues, and plays a critical role in wound ...
cells and human keratinocyte
Keratinocytes are the primary type of Cell (biology), cell found in the epidermis (skin), epidermis, the outermost layer of the skin. In humans, they constitute 90% of epidermal skin cells.
Basal cells in the stratum basale, basal layer (''str ...
s, GCN2 was activated due to UV light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
exposure. The pathways for this activation require further research, although multiple models have been proposed, including crosslinking between GCN2 and tRNA.
PKR
PKR (encoded in humans by the gene ''EIF2AK2'') activation is mainly dependent on the presence ofdouble-stranded RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydr ...
during a viral infection
A viral disease (or viral infection) occurs when an organism's body is invaded by pathogenic viruses, and infectious virus particles (virions) attach to and enter susceptible cells.
Structural Characteristics
Basic structural characteristics, s ...
. dsRNA causes PKR to form dimers, resulting in autophosphorylation and activation. Once activated, PKR will phosphorylate eIF2α which causes a cascade of events that result in viral and host protein synthesis being inhibited. Other stressors that cause the activation of PKR include oxidative stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily Detoxification, detoxify the reactive intermediates or to repair the resulting damage. Disturbances ...
, endoplasmic reticulum stress, growth factor deprivation and bacterial infection
Pathogenic bacteria are bacteria that can cause disease. This article focuses on the bacteria that are pathogenic to humans. Most species of bacteria are harmless and are often beneficial but others can cause infectious diseases. The number of ...
. Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cystei ...
activity early on in apoptosis has also been observed to trigger activation of PKR. However, these stressors differ in that they activate PKR without using dsRNA.
ATF4
When a cell is subjected to stressful conditions, theATF4
Activating transcription factor 4 (tax-responsive enhancer element B67), also known as ATF4, is a protein that in humans is encoded by the ''ATF4'' gene.
Function
This gene encodes a transcription factor that was originally identified as a wi ...
gene is expressed. The ATF4 transcription factor has the ability to form dimers with many different proteins that influence gene expression and cell fate. ATF4 binds to C/EBP‐ATF response element (CARE) sequences which work together to increase the transcription of stress-responsive genes. However, when undergoing amino acid starvation, the sequences will act as amino acid response elements instead.
ATF4 will work together with other transcription factors, such as CHOP and ATF3
Cyclic AMP-dependent transcription factor ATF-3 is a protein that, in humans, is encoded by the ''ATF3'' gene.
Function
Activating transcription factor 3 is a member of the mammalian activation transcription factor/cAMP responsive element-bind ...
, by forming homodimers or heterodimers, resulting in numerous observed effects. The proteins that ATF4 interacts with determines the outcome of the cell during the integrated stress response. For example, ATF4 and ATF3 work to establish homeostasis inside of the cell following stressful conditions. On the other hand, ATF4 and CHOP work together to induce cell death, as well as regulating amino acid biosynthesis, transport and metabolic processes. The presence of a leucine zipper domain (bZIP
The Basic Leucine Zipper Domain (bZIP domain) is found in many DNA binding eukaryotic proteins. One part of the domain contains a region that mediates sequence specific DNA binding properties and the leucine zipper that is required to hold tog ...
) allows ATF4 to work together with many other proteins, thus creating specific responses to different types of stressors. When a cell is undergoing the stress of hypoxia, ATF4 will interact with PHD1 and PHD3 to decrease its transcriptional activity. In addition, when a cell is undergoing amino acid starvation or endoplasmic reticulum stress, TRIP3 also interacts with ATF4 to decrease activity.
One result of ATF4 and stress-response proteins expression is the induction of autophagy
Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent re ...
. During this process, the cell forms autophagosome
An autophagosome is a spherical structure with double layer membranes. It is the key structure in macroautophagy, the intracellular degradation system for cytoplasmic contents (e.g., abnormal intracellular proteins, excess or damaged organelles, in ...
s, or double membraned vesicles, that allow for transportation of material throughout the cell. These autophagosomes can carry unneeded organelles and proteins, as well as damaged or harmful components in an attempt by the cell to maintain homeostasis.
Termination of integrated stress response
In order to terminate the integrated stress response, dephosphorylation of eIF2α is required. Theprotein phosphatase 1
Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been fou ...
complex (PP1) aids in the dephosphorylation of eIF2α. This complex contains a PP1 catalytic subunit as well as two regulatory subunits. This complex is negatively regulated by two proteins: growth arrest and DNA damage‐inducible protein (GADD34), also known as PPP1R15A
Protein phosphatase 1 regulatory subunit 15A also known as growth arrest and DNA damage-inducible protein GADD34 is a protein that in humans is encoded by the ''PPP1R15A'' gene.
The Gadd34/MyD116 gene was originally discovered as a member in a s ...
, or constitutive repressor of eIF2α phosphorylation (CReP), also known as PPP1R15B. CReP acts to keep levels of eIF2α phosphorylation low in cells under normal conditions. GADD34 is produced in response to ATF4 and works to increase dephosphorylation of eIF2α. The dephosphorylation of eIF2α results in the return of normal protein synthesis and cellular function. However, dephosphorylation of eIF2α can also facilitate the production of death-inducing proteins in cases where the cell is so severely damaged that normal functioning cannot be restored.
Mutations affecting integrated stress response
Mutations that affect the functioning of the integrated stress response may have debilitating effects on cells. For example, cells lacking the ATF4 gene are unable to elicit proper gene expression in response to stressors. This results in cells exhibiting issues with amino acid transport,glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pero ...
biosynthesis and oxidative stress resistance. When a mutation inhibits the functioning of PERK, endogenous peroxides accumulate when the cell experiences endoplasmic reticulum stress. In mice and humans lacking PERK, there have been observed destruction of secretory cells undergoing high endoplasmic reticulum stress.
See also
*ISRIB
ISRIB (integrated stress response inhibitor) is an experimental drug that reverses the effects of eIF2α phosphorylation with an IC50 of 5 nM. It was discovered in the laboratory of Peter Walter at University of California, San Francisc ...
, integrated stress response inhibitor
References
{{Reflist Cellular processes Eukaryote biology Gene expression Proteins