Interhalogen Compound on:
[Wikipedia]
[Google]
[Amazon]
In
 The interhalogens of form XY have physical properties intermediate between those of the two parent halogens. The
The interhalogens of form XY have physical properties intermediate between those of the two parent halogens. The 

 *
*
 *
*
 All stable hexatomic and octatomic interhalogens involve a heavier halogen combined with five or seven fluorine atoms. Unlike the other halogens, fluorine atoms have high electronegativity and small size which is able to stabilize them.
*
All stable hexatomic and octatomic interhalogens involve a heavier halogen combined with five or seven fluorine atoms. Unlike the other halogens, fluorine atoms have high electronegativity and small size which is able to stabilize them.
*
 *
*
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, an interhalogen compound is a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
which contains two or more different halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
atoms (fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
, chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
, bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
, iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
, or astatine
Astatine is a chemical element; it has Symbol (chemistry), symbol At and atomic number 85. It is the abundance of elements in Earth's crust, rarest naturally occurring element in the Earth's crust, occurring only as the Decay chain, decay product ...
) and no atoms of elements from any other group.
Most interhalogen compounds known are binary (composed of only two distinct elements). Their formulae are generally , where ''n'' = 1, 3, 5 or 7, and X is the less electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
of the two halogens. The value of ''n'' in interhalogens is always odd, because of the odd valence of halogens. They are all prone to hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
, and ionize to give rise to polyhalogen ions. Those formed with astatine have a very short half-life due to astatine being intensely radioactive.
No interhalogen compounds containing three or more different halogens are definitely known, although a few books claim that and have been obtained, and theoretical studies seem to indicate that some compounds in the series are barely stable.
Some interhalogens, such as , , and , are good halogenating agents. is too reactive to generate fluorine. Beyond that, iodine monochloride
Iodine monochloride is an interhalogen compound with the formula . It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar ...
has several applications, including helping to measure the saturation of fats and oils, and as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
for some reactions. A number of interhalogens, including , are used to form polyhalides.
Similar compounds exist with various pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
s, such as the halogen azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
s (, , , and ) and cyanogen
Cyanogen is the chemical compound with the chemical formula, formula . Its structure is . The simplest stable carbon nitride, it is a Transparency and translucency, colorless and highly toxic gas with a pungency, pungent odor. The molecule is a ...
halides (, , , and ).
Types of interhalogens
Diatomic interhalogens
 The interhalogens of form XY have physical properties intermediate between those of the two parent halogens. The
The interhalogens of form XY have physical properties intermediate between those of the two parent halogens. The covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
bond between the two atoms has some ionic character, the less electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
halogen, X, being oxidised and having a partial positive charge. All combinations of fluorine, chlorine, bromine, and iodine that have the above-mentioned general formula are known, but not all are stable. Some combinations of astatine with other halogens are not even known, and those that are known are highly unstable.
*Chlorine monofluoride
Chlorine monofluoride is a volatile interhalogen compound with the chemical formula . It is a colourless gas at room temperature and is stable even at high temperatures. When cooled to −100 °C, ClF condenses as a pale yellow liquid. Many ...
(ClF) is the lightest interhalogen compound. ClF is a colorless gas with a normal boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of −100 °C.
*Bromine monofluoride
Bromine monofluoride is a quite unstable interhalogen compound with the chemical formula BrF. It can be produced through the reaction of bromine trifluoride (or bromine pentafluoride) and bromine. Due to its lability, the compound can be detected ...
(BrF) has not been obtained as a pure compound — it dissociates into the trifluoride and free bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
. It is created according to the following equation:
::Br2(l) + F2(g) → 2 BrF(g)
:Bromine monofluoride dissociates like this:
::3 BrF → Br2 + BrF3


Iodine monofluoride
Iodine monofluoride is an interhalogen compound of iodine and fluorine with formula IF. It is a chocolate-brown solid that decomposes at 0 °C, disproportionation, disproportionating to elemental iodine and iodine pentafluoride:
:5 IF → 2 ...
(IF) is unstable and decomposes at 0 °C, disproportionating into elemental iodine and iodine pentafluoride
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in speci ...
.
*Bromine monochloride
Bromine monochloride, also called bromine(I) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with chemical formula BrCl. It is a very reactive golden yellow gas with boiling point 5 °C and melting point � ...
(BrCl) is a yellow-brown gas with a boiling point of 5 °C.
*Iodine monochloride
Iodine monochloride is an interhalogen compound with the formula . It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar ...
(ICl) exists as red transparent crystals that melt at 27.2 °C to form a choking brownish liquid (similar in appearance and weight to bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
). It reacts with HCl to form the strong acid HICl2. The crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
of iodine monochloride consists of puckered zig-zag chains, with strong interactions between the chains.
*Astatine monochloride211At+Cl2 at room temperature: (AtCl) is made either by the direct combination of gas-phase astatine
Astatine is a chemical element; it has Symbol (chemistry), symbol At and atomic number 85. It is the abundance of elements in Earth's crust, rarest naturally occurring element in the Earth's crust, occurring only as the Decay chain, decay product ...
with chlorine or by the sequential addition of astatine and dichromate ion to an acidic chloride solution.
*Iodine monobromide
Iodine monobromide is an interhalogen compound with the formula IBr. It is a dark red solid that melts near room temperature. Like iodine monochloride, IBr is used in some types of iodometry. It serves as a source of I+. Its Lewis acid propertie ...
(IBr) is made by the direct combination of the elements to form a dark red crystalline solid. It melts at 42 °C and boils at 116 °C to form a partially dissociated vapour.
*Astatine monobromide
Astatine is a chemical element; it has symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's crust, occurring only as the decay product of various heavier elements. All of astatine's isotopes are short-lived ...
(AtBr) is made by the direct combination of astatine with either bromine vapour or an aqueous solution of iodine monobromide.
*Astatine monoiodide
Astatine iodide is an interhalogen compound with the chemical formula At I. It is the second heaviest known interhalogen compound, after iodine tribromide.
Production
Astatine iodide is produced by the direct combination of astatine and iodin ...
(AtI) is made by direct combination of astatine and iodine.
No astatine fluorides have been discovered yet. Their absence has been speculatively attributed to the extreme reactivity of such compounds, including the reaction of an initially formed fluoride with the walls of the glass container to form a non-volatile product. Thus, although the synthesis of an astatine fluoride is thought to be possible, it may require a liquid halogen fluoride solvent, as has already been used for the characterization of radon fluorides.
In addition, there exist analogous molecules involving pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
s, such as the cyanogen halides.
Tetratomic interhalogens
 *
*Chlorine trifluoride
Chlorine trifluoride is an interhalogen compound with the formula . It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room ...
(ClF3) is a colourless gas that condenses to a green liquid, and freezes to a white solid. It is made by reacting chlorine with an excess of fluorine at 250 °C in a nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
tube. It reacts more violently than fluorine, often explosively. The molecule is planar and T-shaped
Many shapes have metaphorical names, i.e., their names are metaphors: these shapes are named after a most common object that has it. For example, "U-shape" is a shape that resembles the letter U, a bell-shaped curve has the shape of the vertical ...
. It is used in the manufacture of uranium hexafluoride
Uranium hexafluoride, sometimes called hex, is the inorganic compound with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactors and nuclear weapons.
Preparation
Uranium dioxide is co ...
.
*Bromine trifluoride
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating a ...
(BrF3) is a yellow-green liquid that conducts electricity — it self-ionises to form rF2sup>+ and rF4sup>−. It reacts with many metals and metal oxides to form similar ionised entities; with other metals, it forms the metal fluoride plus free bromine and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
; and with water, it forms hydrofluoric acid and hydrobromic acid. It is used in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
as a fluorinating agent. It has the same molecular shape as chlorine trifluoride.
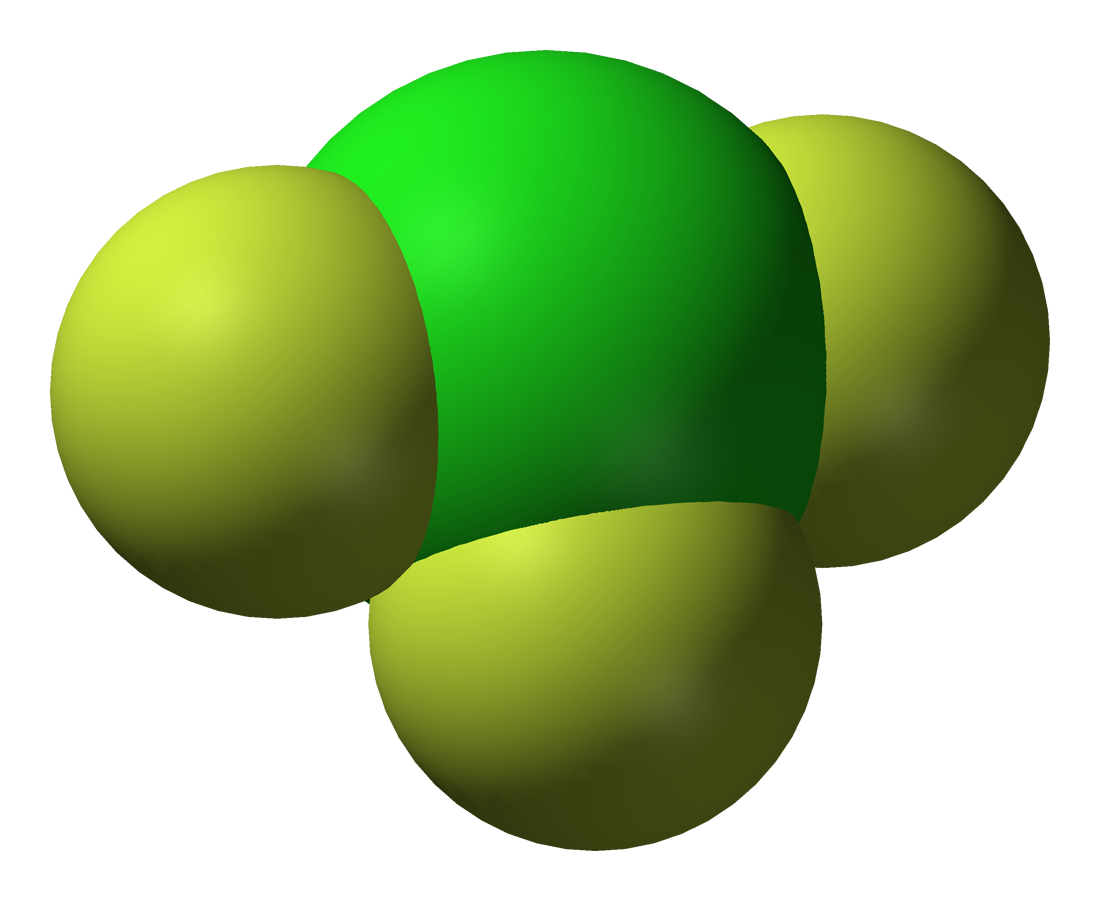
*Iodine trifluoride
Iodine trifluoride is an interhalogen compound with the chemical formula IF3. It is a yellow solid which decomposes above −28 °C. It can be synthesised from the elements, but care must be taken to avoid the formation of IF5.
Reactions
F2 ...
(IF3) is a yellow solid that decomposes above −28 °C. It can be synthesised from the elements, but care must be taken to avoid the formation of IF5. F2 attacks I2 to yield IF3 at −45 °C in CCl3F. Alternatively, at low temperatures, the fluorination reaction
::I2 + 3 XeF2 → 2 IF3 + 3 Xe
:can be used. Not much is known about iodine trifluoride as it is so unstable.
*Iodine trichloride
Iodine trichloride is an interhalogen compound of iodine and chlorine. It is bright yellow but upon time and exposure to light it turns red due to the presence of elemental iodine. In the solid state is present as a planar dimer I2Cl6, with two ...
(ICl3) forms lemon yellow crystals that melt under pressure to a brown liquid. It can be made from the elements at low temperature, or from iodine pentoxide and hydrogen chloride. It reacts with many metal chlorides to form tetrachloroiodides (), and hydrolyses in water. The molecule is a planar dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
** TH-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* ...
(ICl3)2, with each iodine atom surrounded by four chlorine atoms.
* Iodine tribromide (IBr3) is a dark brown liquid.
Hexatomic interhalogens
 All stable hexatomic and octatomic interhalogens involve a heavier halogen combined with five or seven fluorine atoms. Unlike the other halogens, fluorine atoms have high electronegativity and small size which is able to stabilize them.
*
All stable hexatomic and octatomic interhalogens involve a heavier halogen combined with five or seven fluorine atoms. Unlike the other halogens, fluorine atoms have high electronegativity and small size which is able to stabilize them.
*Chlorine pentafluoride
Chlorine pentafluoride is an interhalogen compound with formula . This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its h ...
(ClF5) is a colourless gas, made by reacting chlorine trifluoride with fluorine at high temperatures and high pressures. It reacts violently with water and most metals
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against no ...
and nonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
s.
*Bromine pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.
BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subs ...
(BrF5) is a colourless fuming liquid, made by reacting bromine trifluoride with fluorine at 200 °C. It is physically stable, but decomposes violently on contact with water, organic substances, and most metals and nonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
s.
*Iodine pentafluoride
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in speci ...
(IF5) is a colourless liquid, made by reacting iodine pentoxide with fluorine, or iodine with silver(II) fluoride
Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound - silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.
Preparation
AgF2 can be synthesized by fluori ...
. It is highly reactive, even slowly with glass. It reacts with water to form hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
and with fluorine gas to form iodine heptafluoride
Iodine heptafluoride is an interhalogen compound with the chemical formula I F7. It has an unusual pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement c ...
. The molecule has the form of a tetragonal pyramid.
Octatomic interhalogens
 *
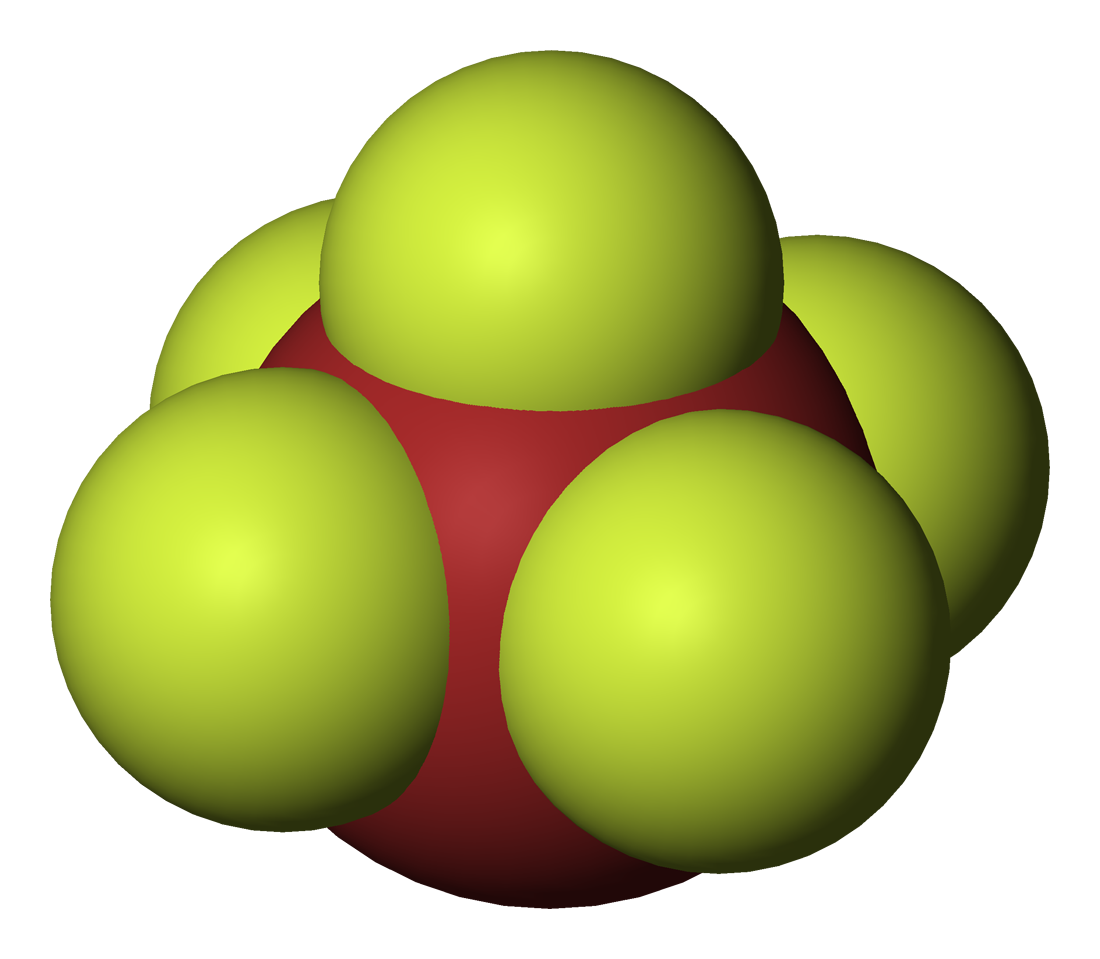
*Iodine heptafluoride
Iodine heptafluoride is an interhalogen compound with the chemical formula I F7. It has an unusual pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement c ...
(IF7) is a colourless gas and a strong fluorinating agent. It is made by reacting iodine pentafluoride with fluorine gas. The molecule is a pentagonal bipyramid
The pentagonal bipyramid (or pentagonal dipyramid) is a polyhedron with ten triangular faces. It is constructed by attaching two pentagonal pyramids to each of their bases. If the triangular faces are equilateral, the pentagonal bipyramid is an ...
. This compound is the only known interhalogen compound where the larger atom is carrying seven of the smaller atoms.
*All attempts to synthesize bromine or chlorine heptafluoride have met with failure; instead, bromine pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.
BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subs ...
or chlorine pentafluoride
Chlorine pentafluoride is an interhalogen compound with formula . This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its h ...
is produced, along with fluorine gas.
Properties
Typically, interhalogen bonds are more reactive than diatomic halogen bonds, because interhalogen bonds are weaker than diatomic halogen bonds, except for F2. If interhalogens are exposed to water, they convert tohalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
and oxyhalide
In chemistry, oxohalides or oxyhalides are a group of chemical compounds with the chemical formula , where X is a halogen, and A is an element different than O and X. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine ( ...
ions. With BrF5, this reaction can be explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An ex ...
. If interhalogens are exposed to silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
, or metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
oxides, then silicon or metal respectively bond with one of the types of halogen, leaving free diatomic halogens and diatomic oxygen. Most interhalogens are halogen fluorides, and all but three (IBr, AtBr, and AtI) of the remainder are halogen chlorides. Chlorine and bromine can each bond to five fluorine atoms, and iodine can bond to seven. AX and AX3 interhalogens can form between two halogens whose electronegativities are relatively close to one another. When interhalogens are exposed to metals, they react to form metal halides of the constituent halogens. The oxidation power of an interhalogen increases with the number of halogens attached to the central atom of the interhalogen, as well as with the decreasing size of the central atom of the compound. Interhalogens containing fluorine are more likely to be volatile than interhalogens containing heavier halogens.
Interhalogens with one or three halogens bonded to a central atom are formed by two elements whose electronegativities are not far apart. Interhalogens with five or seven halogens bonded to a central atom are formed by two elements whose sizes are very different. The number of smaller halogens that can bond to a large central halogen is guided by the ratio of the atomic radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
of the larger halogen over the atomic radius of the smaller halogen. A number of interhalogens, such as IF7, react with all metals except for those in the platinum group
The platinum-group metals (PGMs) are six noble, precious metallic elements clustered together in the periodic table. These elements are all transition metals in the d-block (groups 8, 9, and 10, periods 5 and 6).
The six platinum-group ...
. IF7, unlike interhalogens in the XY5 series, does not react with the fluorides of the alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
.
ClF3 is the most reactive of the XY3 interhalogens. ICl3 is the least reactive. BrF3 has the highest thermal stability of the interhalogens with four atoms. ICl3 has the lowest. Chlorine trifluoride has a boiling point of −12 °C. Bromine trifluoride has a boiling point of 127 °C and is a liquid at room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
. Iodine trichloride melts at 101 °C.
Most interhalogens are covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
gases. Some interhalogens, especially those containing bromine, are liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
s, and most iodine-containing interhalogens are solids. Most of the interhalogens composed of lighter halogens are fairly colorless, but the interhalogens containing heavier halogens are deeper in color due to their higher molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
. In this respect, the interhalogens are similar to the halogens. The greater the difference between the electronegativities of the two halogens in an interhalogen, the higher the boiling point of the interhalogen. All interhalogens are diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
. The bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
of interhalogens in the XY series increases with the size of the constituent halogens. For instance, ClF has a bond length of 1.628 Å, and IBr has a bond length of 2.47 Å.
Production
It is possible to produce larger interhalogens, such as ClF3, by exposing smaller interhalogens, such as ClF, to pure diatomic halogens, such as F2. This method of production is especially useful for generating halogenfluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
s. At temperatures of 250 to 300 °C, this type of production method can also convert larger interhalogens into smaller ones. It is also possible to produce interhalogens by combining two pure halogens at various conditions. This method can generate any interhalogen save for IF7.
Smaller interhalogens, such as ClF, can form by direct reaction with pure halogens. For instance, F2 reacts with Cl2 at 250 °C to form two molecules of ClF. Br2 reacts with diatomic fluorine in the same way, but at 60 °C. I2 reacts with diatomic fluorine at only 35 °C. ClF and BrF can both be produced by the reaction of a larger interhalogen, such as ClF3 or BrF3 and a diatomic molecule of the element lower in the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. Among the hexatomic interhalogens, IF5 has a higher boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
(97 °C) than BrF5 (40.5 °C), although both compounds are liquids at room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
. The interhalogen IF7 can be formed by reacting palladium iodide with fluorine.
See also
* Interchalcogen *Hydrogen halide
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, astatine, or ...
Notes
References
Bibliography
* * *External links
{{Authority control