Ice Hockey Competitions In British Columbia on:
[Wikipedia]
[Google]
[Amazon]
Ice is water
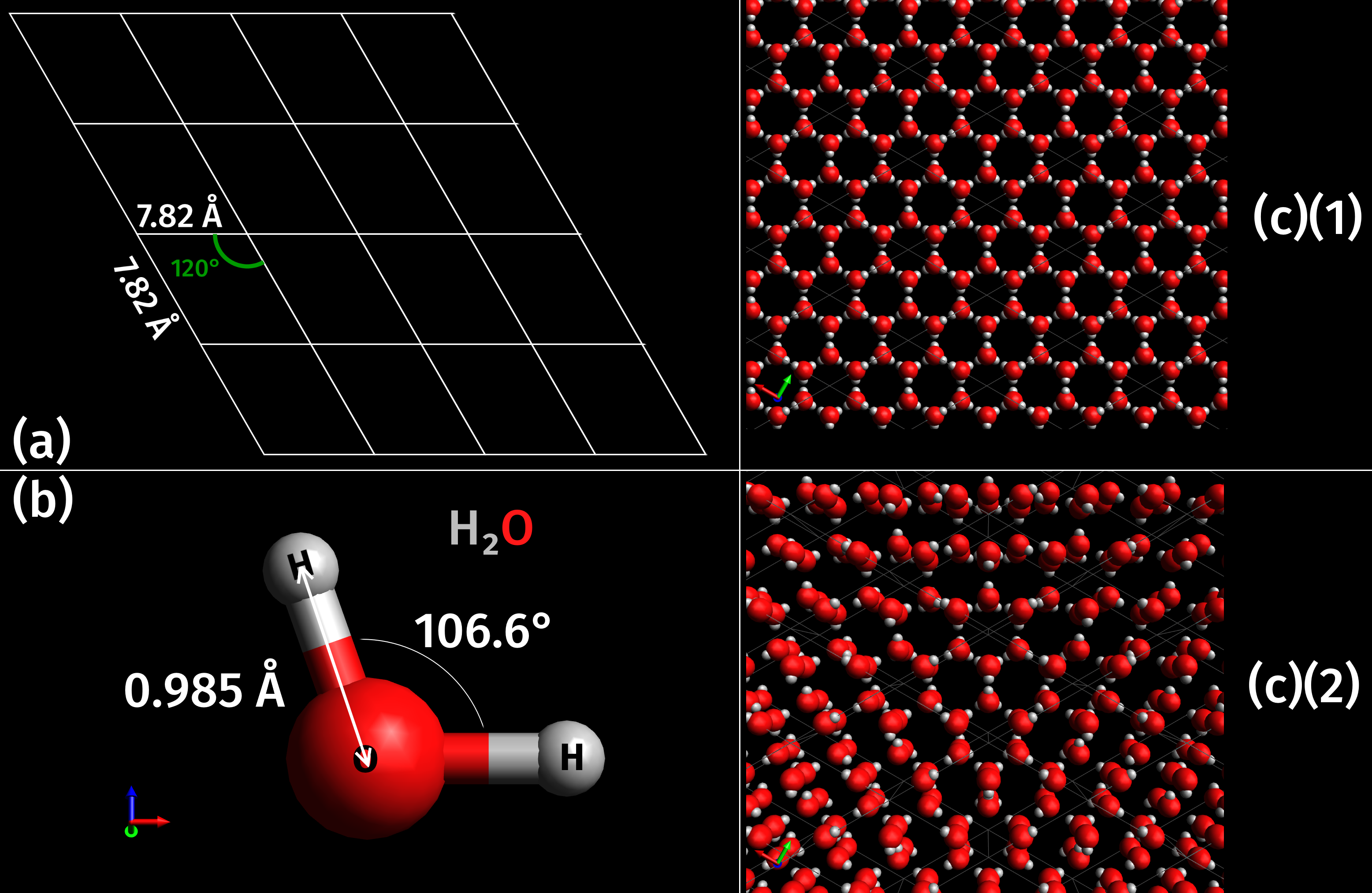 As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. It possesses a regular crystalline structure based on the molecule of water, which consists of a single oxygen atom covalently bonded to two
As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. It possesses a regular crystalline structure based on the molecule of water, which consists of a single oxygen atom covalently bonded to two


 The low coefficient of friction (" slipperiness") of ice has been attributed to the pressure of an object coming into contact with the ice, melting a thin layer of the ice and allowing the object to glide across the surface. For example, the blade of an ice skate, upon exerting pressure on the ice, would melt a thin layer, providing lubrication between the ice and the blade. This explanation, called " pressure melting", originated in the 19th century. However, it does not account for skating on ice temperatures lower than , which is often skated upon. Also, the effect of pressure melting is too small to account for the reduced friction as commonly experienced.
A second theory describing the coefficient of friction of ice suggested that ice molecules at the interface cannot properly bond with the molecules of the mass of ice beneath (and thus are free to move like molecules of liquid water). These molecules remain in a semi-liquid state, providing lubrication regardless of pressure against the ice exerted by any object. However, the significance of this hypothesis is disputed by experiments showing a high coefficient of friction for ice using
The low coefficient of friction (" slipperiness") of ice has been attributed to the pressure of an object coming into contact with the ice, melting a thin layer of the ice and allowing the object to glide across the surface. For example, the blade of an ice skate, upon exerting pressure on the ice, would melt a thin layer, providing lubrication between the ice and the blade. This explanation, called " pressure melting", originated in the 19th century. However, it does not account for skating on ice temperatures lower than , which is often skated upon. Also, the effect of pressure melting is too small to account for the reduced friction as commonly experienced.
A second theory describing the coefficient of friction of ice suggested that ice molecules at the interface cannot properly bond with the molecules of the mass of ice beneath (and thus are free to move like molecules of liquid water). These molecules remain in a semi-liquid state, providing lubrication regardless of pressure against the ice exerted by any object. However, the significance of this hypothesis is disputed by experiments showing a high coefficient of friction for ice using
 The term that collectively describes all of the parts of the Earth's surface where water is in frozen form is the '' cryosphere.'' Ice is an important component of the global climate, particularly in regard to the water cycle. Glaciers and snowpacks are an important storage mechanism for fresh water; over time, they may sublimate or melt. Snowmelt is an important source of seasonal fresh water. The World Meteorological Organization defines several kinds of ice depending on origin, size, shape, influence and so on."WMO SEA-ICE NOMENCLATURE"
The term that collectively describes all of the parts of the Earth's surface where water is in frozen form is the '' cryosphere.'' Ice is an important component of the global climate, particularly in regard to the water cycle. Glaciers and snowpacks are an important storage mechanism for fresh water; over time, they may sublimate or melt. Snowmelt is an important source of seasonal fresh water. The World Meteorological Organization defines several kinds of ice depending on origin, size, shape, influence and so on."WMO SEA-ICE NOMENCLATURE"
Multi-language
) ''World Meteorological Organization'' / '' Arctic and Antarctic Research Institute''. Retrieved 8 April 2012.
 Ice on land ranges from the largest type called an " ice sheet" to smaller
Ice on land ranges from the largest type called an " ice sheet" to smaller
 Ice which forms on moving water tends to be less uniform and stable than ice which forms on calm water. Ice jams (sometimes called "ice dams"), when broken chunks of ice pile up, are the greatest ice hazard on rivers. Ice jams can cause flooding, damage structures in or near the river, and damage vessels on the river. Ice jams can cause some hydropower industrial facilities to completely shut down. An ice dam is a blockage from the movement of a glacier which may produce a proglacial lake. Heavy ice flows in rivers can also damage vessels and require the use of an icebreaker to keep navigation possible.
Ice which forms on moving water tends to be less uniform and stable than ice which forms on calm water. Ice jams (sometimes called "ice dams"), when broken chunks of ice pile up, are the greatest ice hazard on rivers. Ice jams can cause flooding, damage structures in or near the river, and damage vessels on the river. Ice jams can cause some hydropower industrial facilities to completely shut down. An ice dam is a blockage from the movement of a glacier which may produce a proglacial lake. Heavy ice flows in rivers can also damage vessels and require the use of an icebreaker to keep navigation possible.

 Like other precipitation, hail forms in storm clouds when
Like other precipitation, hail forms in storm clouds when
 Snow crystals form when tiny
Snow crystals form when tiny
 There were thriving industries in 16th–17th century England whereby low-lying areas along the Thames Estuary were flooded during the winter, and ice harvested in carts and stored inter-seasonally in insulated wooden houses as a provision to an icehouse often located in large country houses, and widely used to keep fish fresh when caught in distant waters. This was allegedly copied by an Englishman who had seen the same activity in China. Ice was imported into England from Norway on a considerable scale as early as 1823.
In the United States, the first cargo of ice was sent from New York City to
There were thriving industries in 16th–17th century England whereby low-lying areas along the Thames Estuary were flooded during the winter, and ice harvested in carts and stored inter-seasonally in insulated wooden houses as a provision to an icehouse often located in large country houses, and widely used to keep fish fresh when caught in distant waters. This was allegedly copied by an Englishman who had seen the same activity in China. Ice was imported into England from Norway on a considerable scale as early as 1823.
In the United States, the first cargo of ice was sent from New York City to
 Ice is now produced on an industrial scale, for uses including food storage and processing, chemical manufacturing, concrete mixing and curing, and consumer or packaged ice. ASHRAE. "Ice Manufacture". ''2006 ASHRAE Handbook: Refrigeration.'' Inch-Pound Edition. . Most commercial icemakers produce three basic types of fragmentary ice: flake, tubular and plate, using a variety of techniques. Large batch ice makers can produce up to 75 tons of ice per day. In 2002, there were 426 commercial ice-making companies in the United States, with a combined value of shipments of $595,487,000. Home refrigerators can also make ice with a built in icemaker, which will typically make
Ice is now produced on an industrial scale, for uses including food storage and processing, chemical manufacturing, concrete mixing and curing, and consumer or packaged ice. ASHRAE. "Ice Manufacture". ''2006 ASHRAE Handbook: Refrigeration.'' Inch-Pound Edition. . Most commercial icemakers produce three basic types of fragmentary ice: flake, tubular and plate, using a variety of techniques. Large batch ice makers can produce up to 75 tons of ice per day. In 2002, there were 426 commercial ice-making companies in the United States, with a combined value of shipments of $595,487,000. Home refrigerators can also make ice with a built in icemaker, which will typically make
 Ice forming on roads is a dangerous winter hazard. Black ice is very difficult to see, because it lacks the expected frosty surface. Whenever there is freezing rain or snow which occurs at a temperature near the melting point, it is common for ice to build up on the windows of vehicles. Driving safely requires the removal of the ice build-up.
Ice forming on roads is a dangerous winter hazard. Black ice is very difficult to see, because it lacks the expected frosty surface. Whenever there is freezing rain or snow which occurs at a temperature near the melting point, it is common for ice to build up on the windows of vehicles. Driving safely requires the removal of the ice build-up.
 For ships, ice presents two distinct hazards. First, spray and freezing rain can produce an ice build-up on the superstructure of a vessel sufficient to make it unstable, and to require it to be hacked off or melted with steam hoses. Second,
For ships, ice presents two distinct hazards. First, spray and freezing rain can produce an ice build-up on the superstructure of a vessel sufficient to make it unstable, and to require it to be hacked off or melted with steam hoses. Second,
 For aircraft, ice can cause a number of dangers. As an aircraft climbs, it passes through air layers of different temperature and humidity, some of which may be conducive to ice formation. If ice forms on the wings or control surfaces, this may adversely affect the flying qualities of the aircraft. During the first non-stop flight across the Atlantic, the British aviators Captain John Alcock and Lieutenant Arthur Whitten Brown encountered such icing conditions – Brown left the cockpit and climbed onto the wing several times to remove ice which was covering the engine air intakes of the Vickers Vimy aircraft they were flying.
One vulnerability effected by icing that is associated with reciprocating internal combustion engines is the
For aircraft, ice can cause a number of dangers. As an aircraft climbs, it passes through air layers of different temperature and humidity, some of which may be conducive to ice formation. If ice forms on the wings or control surfaces, this may adversely affect the flying qualities of the aircraft. During the first non-stop flight across the Atlantic, the British aviators Captain John Alcock and Lieutenant Arthur Whitten Brown encountered such icing conditions – Brown left the cockpit and climbed onto the wing several times to remove ice which was covering the engine air intakes of the Vickers Vimy aircraft they were flying.
One vulnerability effected by icing that is associated with reciprocating internal combustion engines is the
 Ice also plays a central role in winter recreation and in many sports such as ice skating, tour skating, ice hockey,
Ice also plays a central role in winter recreation and in many sports such as ice skating, tour skating, ice hockey,
 * Engineers used the substantial strength of pack ice when they constructed Antarctica's first floating ice pier in 1973. Such ice piers are used during cargo operations to load and offload ships. Fleet operations personnel make the floating pier during the winter. They build upon naturally occurring frozen seawater in McMurdo Sound until the dock reaches a depth of about . Ice piers have a lifespan of three to five years.
* Engineers used the substantial strength of pack ice when they constructed Antarctica's first floating ice pier in 1973. Such ice piers are used during cargo operations to load and offload ships. Fleet operations personnel make the floating pier during the winter. They build upon naturally occurring frozen seawater in McMurdo Sound until the dock reaches a depth of about . Ice piers have a lifespan of three to five years. * Structures and ice sculptures are built out of large chunks of ice or by spraying waterMakkonen, L. (1994) "Ice and Construction". E & FN Spon, London. . The structures are mostly ornamental (as in the case with ice castles), and not practical for long-term habitation. Ice hotels exist on a seasonal basis in a few cold areas.
* Structures and ice sculptures are built out of large chunks of ice or by spraying waterMakkonen, L. (1994) "Ice and Construction". E & FN Spon, London. . The structures are mostly ornamental (as in the case with ice castles), and not practical for long-term habitation. Ice hotels exist on a seasonal basis in a few cold areas.
Webmineral listing for Ice
Estimating the bearing capacity of ice
{{Authority control Glaciology Minerals Transparent materials Articles containing video clips Limnology Oceanography Cryosphere
frozen
Frozen may refer to:
* the result of freezing
* a paralysis response in extreme cases of fear
Films
* ''Frozen'' (1997 film), a film by Wang Xiaoshuai
* ''Frozen'' (2005 film), a film by Juliet McKoen
* ''Frozen'' (2007 film), a film by Sh ...
into a solid state, typically forming at or below temperatures of 0 degrees Celsius
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The ...
or Depending on the presence of impurities
In chemistry and materials science, impurities are chemical substances inside a confined amount of liquid, gas, or solid, which differ from the chemical composition of the material or compound. Firstly, a pure chemical should appear thermodynam ...
such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color.
In the Solar System, ice is abundant and occurs naturally from as close to the Sun as Mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
to as far away as the Oort cloud objects. Beyond the Solar System, it occurs as interstellar ice. It is abundant on Earth's surfaceparticularly in the polar regions and above the snow line
The climatic snow line is the boundary between a snow-covered and snow-free surface. The actual snow line may adjust seasonally, and be either significantly higher in elevation, or lower. The permanent snow line is the level above which snow wil ...
and, as a common form of precipitation and deposition, plays a key role in Earth's water cycle and climate. It falls as snowflakes and hail or occurs as frost, icicles or ice spike
An ice spike is an ice formation, often in the shape of an inverted icicle, that projects upwards from the surface of a body of frozen water. Ice spikes created by natural processes on the surface of small bodies of frozen water have been repor ...
s and aggregates from snow as glaciers
A glacier (; ) is a persistent body of dense ice that is constantly moving under its own weight. A glacier forms where the accumulation of snow exceeds its ablation over many years, often centuries. It acquires distinguishing features, such as ...
and ice sheets.
Ice exhibits at least eighteen phases ( packing geometries), depending on temperature and pressure. When water is cooled rapidly ( quenching), up to three types of amorphous ice can form depending on its history of pressure and temperature. When cooled slowly, correlated proton tunneling occurs below (, ) giving rise to macroscopic quantum phenomena. Virtually all ice on Earth's surface and in its atmosphere is of a hexagonal crystalline structure denoted as ice Ih (spoken as "ice one h") with minute traces of cubic ice, denoted as ice Ic and, more recently found, Ice VII inclusions in diamonds. The most common phase transition to ice Ih occurs when liquid water is cooled below (, ) at standard atmospheric pressure. It may also be deposited directly by water vapor, as happens in the formation of frost. The transition from ice to water is melting and from ice directly to water vapor is sublimation
Sublimation or sublimate may refer to:
* ''Sublimation'' (album), by Canvas Solaris, 2004
* Sublimation (phase transition), directly from the solid to the gas phase
* Sublimation (psychology), a mature type of defense mechanism
* Sublimate of mer ...
.
Ice is used in a variety of ways, including for cooling, for winter sports, and ice sculpting
Ice sculpture is a form of sculpture that uses ice as the raw material. Sculptures from ice can be abstract or realistic and can be functional or purely decorative. Ice sculptures are generally associated with special or extravagant events because ...
.
Physical properties
 As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. It possesses a regular crystalline structure based on the molecule of water, which consists of a single oxygen atom covalently bonded to two
As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. It possesses a regular crystalline structure based on the molecule of water, which consists of a single oxygen atom covalently bonded to two hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen consti ...
s, or H–O–H. However, many of the physical properties of water and ice are controlled by the formation of hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s between adjacent oxygen and hydrogen atoms; while it is a weak bond, it is nonetheless critical in controlling the structure of both water and ice.
An unusual property of water is that its solid form—ice frozen at atmospheric pressure—is approximately 8.3% less dense than its liquid form; this is equivalent to a volumetric expansion of 9%. The density of ice is 0.9167–0.9168 g/cm3 at 0 °C and standard atmospheric pressure (101,325 Pa), whereas water has a density of 0.9998–0.999863 g/cm3 at the same temperature and pressure. Liquid water is densest, essentially 1.00 g/cm3, at 4 °C and begins to lose its density as the water molecules begin to form the hexagonal crystals of ice as the freezing point is reached. This is due to hydrogen bonding dominating the intermolecular forces, which results in a packing of molecules less compact in the solid. Density of ice increases slightly with decreasing temperature and has a value of 0.9340 g/cm3 at −180 °C (93 K).
When water freezes, it increases in volume (about 9% for fresh water). The effect of expansion during freezing can be dramatic, and ice expansion is a basic cause of freeze-thaw weathering of rock in nature and damage to building foundations and roadways from frost heaving. It is also a common cause of the flooding of houses when water pipes burst due to the pressure of expanding water when it freezes.
The result of this process is that ice (in its most common form) floats on liquid water, which is an important feature in Earth's biosphere. It has been argued that without this property, natural bodies of water would freeze, in some cases permanently, from the bottom up, resulting in a loss of bottom-dependent animal and plant life in fresh and sea water. Sufficiently thin ice sheets allow light to pass through while protecting the underside from short-term weather extremes such as wind chill
Wind chill or windchill (popularly wind chill factor) is the lowering of body temperature due to the passing-flow of lower-temperature air.
Wind chill numbers are always lower than the air temperature for values where the formula is valid. When ...
. This creates a sheltered environment for bacterial and algal colonies. When sea water freezes, the ice is riddled with brine-filled channels which sustain sympagic organisms such as bacteria, algae, copepods and annelids, which in turn provide food for animals such as krill and specialised fish like the bald notothen
The bald notothen (''Pagothenia borchgrevinki''), also known as the bald rockcod, is a species of marine ray-finned fish belonging to the family Nototheniidae, the notothens or cod icefishes. It is native to the Southern Ocean.
Taxonomy
The bald ...
, fed upon in turn by larger animals such as emperor penguins and minke whales.
When ice melts, it absorbs as much energy as it would take to heat an equivalent mass of water by 80 °C. During the melting process, the temperature remains constant at 0 °C. While melting, any energy added breaks the hydrogen bonds between ice (water) molecules. Energy becomes available to increase the thermal energy (temperature) only after enough hydrogen bonds are broken that the ice can be considered liquid water. The amount of energy consumed in breaking hydrogen bonds in the transition from ice to water is known as the '' heat of fusion''.
As with water, ice absorbs light at the red end of the spectrum preferentially as the result of an overtone of an oxygen–hydrogen (O–H) bond stretch. Compared with water, this absorption is shifted toward slightly lower energies. Thus, ice appears blue, with a slightly greener tint than liquid water. Since absorption is cumulative, the color effect intensifies with increasing thickness or if internal reflections cause the light to take a longer path through the ice.
Other colors can appear in the presence of light absorbing impurities, where the impurity is dictating the color rather than the ice itself. For instance, iceberg
An iceberg is a piece of freshwater ice more than 15 m long that has broken off a glacier or an ice shelf and is floating freely in open (salt) water. Smaller chunks of floating glacially-derived ice are called "growlers" or "bergy bits". The ...
s containing impurities (e.g., sediments, algae, air bubbles) can appear brown, grey or green.
Because ice in natural environments is usually close to its melting temperature, its hardness shows pronounced temperature variations. At its melting point, ice has a Mohs hardness of 2 or less, but the hardness increases to about 4 at a temperature of and to 6 at a temperature of , the vaporization point of solid carbon dioxide (dry ice).
Phases
Ice may be any one of the 19 known solid crystalline phases of water, or in an amorphous solid state at various densities. Most liquids under increased pressure freeze at ''higher'' temperatures because the pressure helps to hold the molecules together. However, the strong hydrogen bonds in water make it different: for some pressures higher than , water freezes at a temperature ''below'' 0 °C, as shown in the phase diagram below. The melting of ice under high pressures is thought to contribute to the movement of glaciers. Ice, water, and water vapour can coexist at the triple point, which is exactly at a pressure of 611.657 Pa. The kelvin was in fact defined as of the difference between this triple point andabsolute zero
Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibration ...
, though this definition changed
Change or Changing may refer to:
Alteration
* Impermanence, a difference in a state of affairs at different points in time
* Menopause, also referred to as "the change", the permanent cessation of the menstrual period
* Metamorphosis, or change, ...
in May 2019. Unlike most other solids, ice is difficult to superheat. In an experiment, ice at −3 °C was superheated to about 17 °C for about 250 picosecond
A picosecond (abbreviated as ps) is a unit of time in the International System of Units (SI) equal to 10−12 or (one trillionth) of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 ...
s.
Subjected to higher pressures and varying temperatures, ice can form in 19 separate known crystalline phases. With care, at least 15 of these phases (one of the known exceptions being ice X) can be recovered at ambient pressure and low temperature in metastable form. The types are differentiated by their crystalline structure, proton ordering, and density. There are also two metastable phases of ice under pressure, both fully hydrogen-disordered; these are IV and XII
XII may refer to:
* 12 (number) or XII in Roman numerals
* 12th century or XII in Roman numerals
* ''XII'' (album), a 2012 album by American country music singer Neal McCoy
* ''XII'' (single), a 2019 single album by K-pop singer Chungha, featuri ...
. Ice XII was discovered in 1996. In 2006, XIII and XIV were discovered. Ices XI, XIII, and XIV are hydrogen-ordered forms of ices I, V, and XII respectively. In 2009, ice XV was found at extremely high pressures and −143 °C. At even higher pressures, ice is predicted to become a metal; this has been variously estimated to occur at 1.55 TPa or 5.62 TPa.
As well as crystalline forms, solid water can exist in amorphous states as amorphous ice (ASW) of varying densities. Water in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
is dominated by amorphous ice, making it likely the most common form of water in the universe. Low-density ASW (LDA), also known as hyperquenched glassy water, may be responsible for noctilucent clouds on Earth and is usually formed by deposition of water vapor in cold or vacuum conditions. High-density ASW (HDA) is formed by compression of ordinary ice I or LDA at GPa pressures. Very-high-density ASW (VHDA) is HDA slightly warmed to 160 K under 1–2 GPa pressures.
In outer space, hexagonal crystalline ice (the predominant form found on Earth) is extremely rare. Amorphous ice is more common; however, hexagonal crystalline ice can be formed by volcanic action.
Ice from a theorized superionic water may possess two crystalline structures. At pressures in excess of such ''superionic ice'' would take on a body-centered cubic structure. However, at pressures in excess of the structure may shift to a more stable face-centered cubic lattice. It is speculated that superionic ice could compose the interior of ice giants such as Uranus and Neptune.

Friction properties
atomic force microscopy
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the op ...
.
A third theory is "friction heating", which suggests that friction of the material is the cause of the ice layer melting. However, this theory does not sufficiently explain why ice is slippery when standing still even at below-zero temperatures.
A comprehensive theory of ice friction takes into account all the above-mentioned friction mechanisms. This model allows quantitative estimation of the friction coefficient of ice against various materials as a function of temperature and sliding speed. In typical conditions related to winter sports and tires of a vehicle on ice, melting of a thin ice layer due to the frictional heating is the primary reason for the slipperiness. The mechanism controlling the frictional properties of ice is still an active area of scientific study.
Natural formation
 The term that collectively describes all of the parts of the Earth's surface where water is in frozen form is the '' cryosphere.'' Ice is an important component of the global climate, particularly in regard to the water cycle. Glaciers and snowpacks are an important storage mechanism for fresh water; over time, they may sublimate or melt. Snowmelt is an important source of seasonal fresh water. The World Meteorological Organization defines several kinds of ice depending on origin, size, shape, influence and so on."WMO SEA-ICE NOMENCLATURE"
The term that collectively describes all of the parts of the Earth's surface where water is in frozen form is the '' cryosphere.'' Ice is an important component of the global climate, particularly in regard to the water cycle. Glaciers and snowpacks are an important storage mechanism for fresh water; over time, they may sublimate or melt. Snowmelt is an important source of seasonal fresh water. The World Meteorological Organization defines several kinds of ice depending on origin, size, shape, influence and so on."WMO SEA-ICE NOMENCLATURE"Multi-language
) ''World Meteorological Organization'' / '' Arctic and Antarctic Research Institute''. Retrieved 8 April 2012.
Clathrate hydrate
Clathrate hydrates, or gas hydrates, clathrates, hydrates, etc., are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped ins ...
s are forms of ice that contain gas molecules trapped within its crystal lattice.
On the oceans
Ice that is found at sea may be in the form of drift ice floating in the water, fast ice fixed to a shoreline or anchor ice if attached to the sea bottom. Ice which calves (breaks off) from an ice shelf or glacier may become an iceberg. Sea ice can be forced together by currents and winds to form pressure ridges up to tall. Navigation through areas of sea ice occurs in openings called " polynyas" or " leads" or requires the use of a special ship called an " icebreaker".On land and structures
 Ice on land ranges from the largest type called an " ice sheet" to smaller
Ice on land ranges from the largest type called an " ice sheet" to smaller ice cap
In glaciology, an ice cap is a mass of ice that covers less than of land area (usually covering a highland area). Larger ice masses covering more than are termed ice sheets.
Description
Ice caps are not constrained by topographical features ...
s and ice fields to glaciers and ice streams to the snow line
The climatic snow line is the boundary between a snow-covered and snow-free surface. The actual snow line may adjust seasonally, and be either significantly higher in elevation, or lower. The permanent snow line is the level above which snow wil ...
and snow fields.
Aufeis is layered ice that forms in Arctic and subarctic stream valleys. Ice, frozen in the stream bed, blocks normal groundwater discharge, and causes the local water table to rise, resulting in water discharge on top of the frozen layer. This water then freezes, causing the water table to rise further and repeat the cycle. The result is a stratified ice deposit, often several meters thick.
Freezing rain is a type of winter storm called an ice storm where rain falls and then freezes producing a glaze of ice. Ice can also form icicles, similar to stalactite
A stalactite (, ; from the Greek 'stalaktos' ('dripping') via
''stalassein'' ('to drip') is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble an ...
s in appearance, or stalagmite
A stalagmite (, ; from the Greek , from , "dropping, trickling")
is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically ...
-like forms as water drips and re-freezes.
The term "ice dam" has three meanings (others discussed below). On structures, an ice dam is the buildup of ice on a sloped roof which stops melt water from draining properly and can cause damage from water leaks in buildings.
On rivers and streams
Ice disc
Ice discs, ice circles, ice pans, ice pancakes or ice crepes are a very rare natural phenomenon that occurs in slow moving water in cold climates. They are thin circular slabs of ice that rotate slowly on a body of water's surface.
Types
Ice ...
s are circular formations of ice surrounded by water in a river.
Pancake ice is a formation of ice generally created in areas with less calm conditions.
On lakes
Ice forms on calm water from the shores, a thin layer spreading across the surface, and then downward. Ice on lakes is generally four types: primary, secondary, superimposed and agglomerate. Primary ice forms first. Secondary ice forms below the primary ice in a direction parallel to the direction of the heat flow. Superimposed ice forms on top of the ice surface from rain or water which seeps up through cracks in the ice which often settles when loaded with snow. Shelf ice occurs when floating pieces of ice are driven by the wind piling up on the windward shore. Candle ice is a form of rotten ice that develops in columns perpendicular to the surface of a lake. An ice shove occurs when ice movement, caused by ice expansion and/or wind action, occurs to the extent that ice pushes onto the shores of lakes, often displacing sediment that makes up the shoreline.In the air
Rime
Rime is a type of ice formed on cold objects when drops of water crystallize on them. This can be observed infog
Fog is a visible aerosol consisting of tiny water droplets or ice crystals suspended in the air at or near the Earth's surface. Reprint from Fog can be considered a type of low-lying cloud usually resembling stratus, and is heavily influ ...
gy weather, when the temperature drops during the night. Soft rime
Rime ice forms when supercooled water liquid droplets freeze onto surfaces. Meteorologists distinguish between three basic types of ice forming on vertical and horizontal surfaces by deposition of supercooled water droplets. There are also interm ...
contains a high proportion of trapped air, making it appear white rather than transparent, and giving it a density about one quarter of that of pure ice. Hard rime is comparatively dense.
Pellets

Ice pellets
Ice pellets are a form of precipitation consisting of small, hard, translucent balls of ice. Ice pellets are different from graupel ("soft hail") which is made of frosty white opaque rime, and from a mixture of rain and snow which is a slushy ...
are a form of precipitation consisting of small, translucent balls of ice. This form of precipitation is also referred to as "sleet" by the United States National Weather Service. (In British English "sleet" refers to a mixture of rain and snow.) Ice pellets are usually smaller than hailstones. They often bounce when they hit the ground, and generally do not freeze into a solid mass unless mixed with freezing rain. The METAR
METAR is a format for reporting weather information. A METAR weather report is predominantly used by aircraft pilots, and by meteorologists, who use aggregated METAR information to assist in weather forecasting.
Raw METAR is the most common form ...
code for ice pellets is ''PL''.
Ice pellets form when a layer of above-freezing air is located between above the ground, with sub-freezing air both above and below it. This causes the partial or complete melting of any snowflakes falling through the warm layer. As they fall back into the sub-freezing layer closer to the surface, they re-freeze into ice pellets. However, if the sub-freezing layer beneath the warm layer is too small, the precipitation will not have time to re-freeze, and freezing rain will be the result at the surface. A temperature profile showing a warm layer above the ground is most likely to be found in advance of a warm front during the cold season, but can occasionally be found behind a passing cold front.
Hail
 Like other precipitation, hail forms in storm clouds when
Like other precipitation, hail forms in storm clouds when supercooled
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
water droplets freeze on contact with condensation nuclei
Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 µm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This ca ...
, such as dust or dirt. The storm's updraft blows the hailstones to the upper part of the cloud. The updraft dissipates and the hailstones fall down, back into the updraft, and are lifted up again. Hail has a diameter of or more. Within METAR
METAR is a format for reporting weather information. A METAR weather report is predominantly used by aircraft pilots, and by meteorologists, who use aggregated METAR information to assist in weather forecasting.
Raw METAR is the most common form ...
code, GR is used to indicate larger hail, of a diameter of at least and GS for smaller. Stones just larger than golf ball-sized are one of the most frequently reported hail sizes. Hailstones can grow to and weigh more than . In large hailstones, latent heat released by further freezing may melt the outer shell of the hailstone. The hailstone then may undergo 'wet growth', where the liquid outer shell collects other smaller hailstones. The hailstone gains an ice layer and grows increasingly larger with each ascent. Once a hailstone becomes too heavy to be supported by the storm's updraft, it falls from the cloud.
Hail forms in strong thunderstorm clouds, particularly those with intense updrafts, high liquid water content, great vertical extent, large water droplets, and where a good portion of the cloud layer is below freezing . Hail-producing clouds are often identifiable by their green coloration. The growth rate is maximized at about , and becomes vanishingly small much below as supercooled water droplets become rare. For this reason, hail is most common within continental interiors of the mid-latitudes, as hail formation is considerably more likely when the freezing level is below the altitude of . Entrainment of dry air into strong thunderstorms over continents can increase the frequency of hail by promoting evaporational cooling which lowers the freezing level of thunderstorm clouds giving hail a larger volume to grow in. Accordingly, hail is actually less common in the tropics despite a much higher frequency of thunderstorms than in the mid-latitudes because the atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A s ...
over the tropics tends to be warmer over a much greater depth. Hail in the tropics occurs mainly at higher elevations.
Snow
 Snow crystals form when tiny
Snow crystals form when tiny supercool
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
ed cloud droplets (about 10 μm
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American spelling), also commonly known as a micron, is a unit of length in the International System of Unit ...
in diameter) freeze. These droplets are able to remain liquid at temperatures lower than , because to freeze, a few molecules in the droplet need to get together by chance to form an arrangement similar to that in an ice lattice; then the droplet freezes around this "nucleus". Experiments show that this "homogeneous" nucleation of cloud droplets only occurs at temperatures lower than . In warmer clouds an aerosol particle or "ice nucleus" must be present in (or in contact with) the droplet to act as a nucleus. Our understanding of what particles make efficient ice nuclei is poor – what we do know is they are very rare compared to that cloud condensation nuclei on which liquid droplets form. Clays, desert dust and biological particles may be effective, although to what extent is unclear. Artificial nuclei are used in cloud seeding
Cloud seeding is a type of weather modification that aims to change the amount or type of precipitation that falls from clouds by dispersing substances into the air that serve as cloud condensation or ice nuclei, which alter the microphysical p ...
. The droplet then grows by condensation of water vapor onto the ice surfaces.
Diamond dust
So-called "diamond dust", also known as ice needles or ice crystals, forms at temperatures approaching due to air with slightly higher moisture from aloft mixing with colder, surface-based air. The METAR identifier for diamond dust within international hourly weather reports is ''IC''.Ablation
Ablation of ice refers to both its melting and its dissolution. The melting of ice means entails the breaking ofhydrogen bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
between the water molecules. The ordering of the molecules in the solid breaks down to a less ordered state and the solid melts to become a liquid. This is achieved by increasing the internal energy of the ice beyond the melting point. When ice melts it absorbs as much energy as would be required to heat an equivalent amount of water by 80 °C. While melting, the temperature of the ice surface remains constant at 0 °C. The rate of the melting process depends on the efficiency of the energy exchange process. An ice surface in fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. Although the term specifically excludes seawater and brackish water, it does include ...
melts solely by free convection with a rate that depends linearly on the water temperature, ''T''∞, when ''T''∞ is less than 3.98 °C, and superlinearly when ''T''∞ is equal to or greater than 3.98 °C, with the rate being proportional to (T∞ − 3.98 °C)''α'', with ''α'' = for ''T''∞ much greater than 8 °C, and α = for in between temperatures ''T''∞.
In salty ambient conditions, dissolution rather than melting often causes the ablation of ice. For example, the temperature of the Arctic Ocean is generally below the melting point of ablating sea ice. The phase transition from solid to liquid is achieved by mixing salt and water molecules, similar to the dissolution of sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
in water, even though the water temperature is far below the melting point of the sugar. Thus the dissolution rate is limited by salt transport whereas melting can occur at much higher rates that are characteristic for heat transport
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, ...
.
Role in human activities
Humans have used ice for cooling andfood preservation
Food preservation includes processes that make food more resistant to microorganism growth and slow the oxidation of fats. This slows down the decomposition and rancidification process. Food preservation may also include processes that inhibit ...
for centuries, relying on harvesting natural ice in various forms and then transitioning to the mechanical production of the material. Ice also presents a challenge to transportation in various forms and a setting for winter sports.
Cooling
Ice has long been valued as a means of cooling. In 400 BC Iran, Persian engineers had already mastered the technique of storing ice in the middle of summer in the desert. The ice was brought in during the winters from nearby mountains in bulk amounts, and stored in specially designed, naturally cooled ''refrigerators'', called yakhchal (meaning ''ice storage''). This was a large underground space (up to 5000 m3) that had thick walls (at least two meters at the base) made of a special mortar called '' sarooj'', composed of sand, clay, egg whites, lime, goat hair, and ash in specific proportions, and which was known to be resistant to heat transfer. This mixture was thought to be completely water impenetrable. The space often had access to a qanat, and often contained a system of windcatchers which could easily bring temperatures inside the space down to frigid levels on summer days. The ice was used to chill treats for royalty.Harvesting
 There were thriving industries in 16th–17th century England whereby low-lying areas along the Thames Estuary were flooded during the winter, and ice harvested in carts and stored inter-seasonally in insulated wooden houses as a provision to an icehouse often located in large country houses, and widely used to keep fish fresh when caught in distant waters. This was allegedly copied by an Englishman who had seen the same activity in China. Ice was imported into England from Norway on a considerable scale as early as 1823.
In the United States, the first cargo of ice was sent from New York City to
There were thriving industries in 16th–17th century England whereby low-lying areas along the Thames Estuary were flooded during the winter, and ice harvested in carts and stored inter-seasonally in insulated wooden houses as a provision to an icehouse often located in large country houses, and widely used to keep fish fresh when caught in distant waters. This was allegedly copied by an Englishman who had seen the same activity in China. Ice was imported into England from Norway on a considerable scale as early as 1823.
In the United States, the first cargo of ice was sent from New York City to Charleston, South Carolina
Charleston is the largest city in the U.S. state of South Carolina, the county seat of Charleston County, and the principal city in the Charleston–North Charleston metropolitan area. The city lies just south of the geographical midpoint o ...
, in 1799, and by the first half of the 19th century, ice harvesting had become a big business. Frederic Tudor
Frederic Tudor (September 4, 1783 – February 6, 1864) was an American businessman and merchant. Known as Boston's "Ice King", he was the founder of the Tudor Ice Company and a pioneer of the international ice trade in the early 19th century. H ...
, who became known as the "Ice King", worked on developing better insulation products for long distance shipments of ice, especially to the tropics; this became known as the ice trade.
Trieste sent ice to Egypt, Corfu
Corfu (, ) or Kerkyra ( el, Κέρκυρα, Kérkyra, , ; ; la, Corcyra.) is a Greek island in the Ionian Sea, of the Ionian Islands, and, including its small satellite islands, forms the margin of the northwestern frontier of Greece. The isl ...
, and Zante; Switzerland, to France; and Germany sometimes was supplied from Bavarian lakes. The Hungarian Parliament building used ice harvested in the winter from Lake Balaton for air conditioning.
Ice houses were used to store ice formed in the winter, to make ice available all year long, and an early type of refrigerator
A refrigerator, colloquially fridge, is a commercial and home appliance consisting of a thermally insulated compartment and a heat pump (mechanical, electronic or chemical) that transfers heat from its inside to its external environment so th ...
known as an icebox was cooled using a block of ice placed inside it. In many cities, it was not unusual to have a regular ice delivery service during the summer. The advent of artificial refrigeration technology has since made delivery of ice obsolete.
Ice is still harvested for ice and snow sculpture events. For example, a swing saw is used to get ice for the Harbin International Ice and Snow Sculpture Festival
The Harbin International Ice and Snow festival () is an annual winter festival that takes place with a theme in Harbin, Heilongjiang, China, and now is the largest ice and snow festival in the world. At first participants in the festival were m ...
each year from the frozen surface of the Songhua River.
Mechanical production
 Ice is now produced on an industrial scale, for uses including food storage and processing, chemical manufacturing, concrete mixing and curing, and consumer or packaged ice. ASHRAE. "Ice Manufacture". ''2006 ASHRAE Handbook: Refrigeration.'' Inch-Pound Edition. . Most commercial icemakers produce three basic types of fragmentary ice: flake, tubular and plate, using a variety of techniques. Large batch ice makers can produce up to 75 tons of ice per day. In 2002, there were 426 commercial ice-making companies in the United States, with a combined value of shipments of $595,487,000. Home refrigerators can also make ice with a built in icemaker, which will typically make
Ice is now produced on an industrial scale, for uses including food storage and processing, chemical manufacturing, concrete mixing and curing, and consumer or packaged ice. ASHRAE. "Ice Manufacture". ''2006 ASHRAE Handbook: Refrigeration.'' Inch-Pound Edition. . Most commercial icemakers produce three basic types of fragmentary ice: flake, tubular and plate, using a variety of techniques. Large batch ice makers can produce up to 75 tons of ice per day. In 2002, there were 426 commercial ice-making companies in the United States, with a combined value of shipments of $595,487,000. Home refrigerators can also make ice with a built in icemaker, which will typically make ice cube
An ice cube is a small piece of ice, which is typically rectangular as viewed from above and trapezoidal as viewed from the side. Ice cubes are products of mechanical refrigeration and are usually produced to cool beverages. They may be produc ...
s or crushed ice. Stand-alone icemaker units that make ice cubes are often called ice machines.
Transportation
Ice can present challenges to safe transportation on land, sea and in the air.Land travel
 Ice forming on roads is a dangerous winter hazard. Black ice is very difficult to see, because it lacks the expected frosty surface. Whenever there is freezing rain or snow which occurs at a temperature near the melting point, it is common for ice to build up on the windows of vehicles. Driving safely requires the removal of the ice build-up.
Ice forming on roads is a dangerous winter hazard. Black ice is very difficult to see, because it lacks the expected frosty surface. Whenever there is freezing rain or snow which occurs at a temperature near the melting point, it is common for ice to build up on the windows of vehicles. Driving safely requires the removal of the ice build-up. Ice scraper
An ice scraper is a handheld tool for removing frost, ice, and snow from windows, usually on automobiles. Basic scrapers have a plastic blade and handle, though some have blades made out of metal. More complex models often include brushes to h ...
s are tools designed to break the ice free and clear the windows, though removing the ice can be a long and laborious process.
Far enough below the freezing point, a thin layer of ice crystals can form on the inside surface of windows. This usually happens when a vehicle has been left alone after being driven for a while, but can happen while driving, if the outside temperature is low enough. Moisture from the driver's breath is the source of water for the crystals. It is troublesome to remove this form of ice, so people often open their windows slightly when the vehicle is parked in order to let the moisture dissipate, and it is now common for cars to have rear-window defroster
A defogger, demister, or defroster is a system to clear condensation and thaw frost from the windshield, backglass, or side windows of a motor vehicle. The rear window defroster was invented by German automobile engineer Heinz Kunert.
Types
P ...
s to solve the problem. A similar problem can happen in homes, which is one reason why many colder regions require double-pane windows for insulation.
When the outdoor temperature stays below freezing for extended periods, very thick layers of ice can form on lakes and other bodies of water, although places with flowing water require much colder temperatures. The ice can become thick enough to drive onto with automobiles and trucks. Doing this safely requires a thickness of at least 30 cm (one foot).
Water-borne travel
 For ships, ice presents two distinct hazards. First, spray and freezing rain can produce an ice build-up on the superstructure of a vessel sufficient to make it unstable, and to require it to be hacked off or melted with steam hoses. Second,
For ships, ice presents two distinct hazards. First, spray and freezing rain can produce an ice build-up on the superstructure of a vessel sufficient to make it unstable, and to require it to be hacked off or melted with steam hoses. Second, iceberg
An iceberg is a piece of freshwater ice more than 15 m long that has broken off a glacier or an ice shelf and is floating freely in open (salt) water. Smaller chunks of floating glacially-derived ice are called "growlers" or "bergy bits". The ...
s – large masses of ice floating in water (typically created when glaciers reach the sea) – can be dangerous if struck by a ship when underway. Icebergs have been responsible for the sinking of many ships, the most famous being the ''Titanic''. For harbors near the poles, being ice-free, ideally all year long, is an important advantage. Examples are Murmansk (Russia), Petsamo Petsamo may refer to:
* Petsamo Province, a province of Finland from 1921 to 1922
* Petsamo, Tampere, a district in Tampere, Finland
* Pechengsky District, Russia, formerly known as Petsamo
* Pechenga (urban-type settlement), Murmansk Oblast, Russi ...
(Russia, formerly Finland), and Vardø (Norway). Harbors which are not ice-free are opened up using icebreakers.
Air travel
 For aircraft, ice can cause a number of dangers. As an aircraft climbs, it passes through air layers of different temperature and humidity, some of which may be conducive to ice formation. If ice forms on the wings or control surfaces, this may adversely affect the flying qualities of the aircraft. During the first non-stop flight across the Atlantic, the British aviators Captain John Alcock and Lieutenant Arthur Whitten Brown encountered such icing conditions – Brown left the cockpit and climbed onto the wing several times to remove ice which was covering the engine air intakes of the Vickers Vimy aircraft they were flying.
One vulnerability effected by icing that is associated with reciprocating internal combustion engines is the
For aircraft, ice can cause a number of dangers. As an aircraft climbs, it passes through air layers of different temperature and humidity, some of which may be conducive to ice formation. If ice forms on the wings or control surfaces, this may adversely affect the flying qualities of the aircraft. During the first non-stop flight across the Atlantic, the British aviators Captain John Alcock and Lieutenant Arthur Whitten Brown encountered such icing conditions – Brown left the cockpit and climbed onto the wing several times to remove ice which was covering the engine air intakes of the Vickers Vimy aircraft they were flying.
One vulnerability effected by icing that is associated with reciprocating internal combustion engines is the carburetor
A carburetor (also spelled carburettor) is a device used by an internal combustion engine to control and mix air and fuel entering the engine. The primary method of adding fuel to the intake air is through the venturi tube in the main meteri ...
. As air is sucked through the carburetor into the engine, the local air pressure is lowered, which causes adiabatic cooling. Thus, in humid near-freezing conditions, the carburetor will be colder, and tend to ice up. This will block the supply of air to the engine, and cause it to fail. For this reason, aircraft reciprocating engines with carburetors are provided with carburetor air intake heaters. The increasing use of fuel injection
Fuel injection is the introduction of fuel in an internal combustion engine, most commonly automotive engines, by the means of an injector. This article focuses on fuel injection in reciprocating piston and Wankel rotary engines.
All comp ...
—which does not require carburetors—has made "carb icing" less of an issue for reciprocating engines.
Jet engines do not experience carb icing, but recent evidence indicates that they can be slowed, stopped, or damaged by internal icing in certain types of atmospheric conditions much more easily than previously believed. In most cases, the engines can be quickly restarted and flights are not endangered, but research continues to determine the exact conditions which produce this type of icing, and find the best methods to prevent, or reverse it, in flight.
Recreation and sports
 Ice also plays a central role in winter recreation and in many sports such as ice skating, tour skating, ice hockey,
Ice also plays a central role in winter recreation and in many sports such as ice skating, tour skating, ice hockey, bandy
Bandy is a winter sport and ball sport played by two teams wearing ice skates on a large ice surface (either indoors or outdoors) while using sticks to direct a ball into the opposing team's goal. The international governing body for bandy is ...
, ice fishing, ice climbing, curling, broomball and sled racing on bobsled, luge
A luge is a small one- or two-person sled on which one sleds supine (face up) and feet-first. A luger steers by using the calf muscles to flex the sled's runners or by exerting opposite shoulder pressure to the seat. Racing sleds weigh for s ...
and skeleton
A skeleton is the structural frame that supports the body of an animal. There are several types of skeletons, including the exoskeleton, which is the stable outer shell of an organism, the endoskeleton, which forms the support structure inside ...
. Many of the different sports played on ice get international attention every four years during the Winter Olympic Games.
A sort of sailboat on blades gives rise to ice yachting. Another sport is ice racing, where drivers must speed on lake ice, while also controlling the skid of their vehicle (similar in some ways to dirt track racing
Dirt track racing is a form of motorsport held on clay or dirt surfaced oval race tracks often used for thoroughbred horse racing. Dirt track racing started in the United States before World War I and became widespread during the 1920s and 1930s ...
). The sport has even been modified for ice rinks.
Other uses
As thermal ballast
* Ice is used to cool and preserve food in iceboxes. *Ice cube
An ice cube is a small piece of ice, which is typically rectangular as viewed from above and trapezoidal as viewed from the side. Ice cubes are products of mechanical refrigeration and are usually produced to cool beverages. They may be produc ...
s or crushed ice can be used to cool drinks. As the ice melts, it absorbs heat and keeps the drink near .
* Ice can be used as part of an air conditioning system, using battery- or solar-powered fans to blow hot air over the ice. This is especially useful during heat waves when power is out and standard (electrically powered) air conditioners do not work.
* Ice can be used (like other cold pack
An ice pack or gel pack is a portable bag filled with water, refrigerant gel, or liquid, meant to provide cooling. They can be divided into the reusable type, which works as a thermal mass and requires freezing, or the instant type, which cools ...
s) to reduce swelling (by decreasing blood flow) and pain by pressing it against an area of the body.
As structural material
 * Engineers used the substantial strength of pack ice when they constructed Antarctica's first floating ice pier in 1973. Such ice piers are used during cargo operations to load and offload ships. Fleet operations personnel make the floating pier during the winter. They build upon naturally occurring frozen seawater in McMurdo Sound until the dock reaches a depth of about . Ice piers have a lifespan of three to five years.
* Engineers used the substantial strength of pack ice when they constructed Antarctica's first floating ice pier in 1973. Such ice piers are used during cargo operations to load and offload ships. Fleet operations personnel make the floating pier during the winter. They build upon naturally occurring frozen seawater in McMurdo Sound until the dock reaches a depth of about . Ice piers have a lifespan of three to five years. * Structures and ice sculptures are built out of large chunks of ice or by spraying waterMakkonen, L. (1994) "Ice and Construction". E & FN Spon, London. . The structures are mostly ornamental (as in the case with ice castles), and not practical for long-term habitation. Ice hotels exist on a seasonal basis in a few cold areas.
* Structures and ice sculptures are built out of large chunks of ice or by spraying waterMakkonen, L. (1994) "Ice and Construction". E & FN Spon, London. . The structures are mostly ornamental (as in the case with ice castles), and not practical for long-term habitation. Ice hotels exist on a seasonal basis in a few cold areas. Igloo
An igloo (Inuit languages: , Inuktitut syllabics (plural: )), also known as a snow house or snow hut, is a type of shelter built of suitable snow.
Although igloos are often associated with all Inuit, they were traditionally used only b ...
s are another example of a temporary structure, made primarily from snow.
* In cold climates, roads are regularly prepared on iced-over lakes and archipelago areas. Temporarily, even a railroad has been built on ice.
* During World War II, Project Habbakuk
Project Habakkuk or Habbakuk (spelling varies) was a plan by the British during the Second World War to construct an aircraft carrier out of pykrete (a mixture of wood pulp and ice) for use against German U-boats in the mid-Atlantic, which wer ...
was an Allied programme which investigated the use of pykrete (wood fibers mixed with ice) as a possible material for warships, especially aircraft carriers, due to the ease with which a vessel immune to torpedoes, and a large deck, could be constructed by ice. A small-scale prototype was built, but the need for such a vessel in the war was removed prior to building it in full-scale.
* Ice has even been used as the material for a variety of musical instruments, for example by percussionist Terje Isungset.
Non-water
The solid phases of several other volatile substances are also referred to as ''ices''; generally a volatile is classed as an ice if its melting point lies above or around 100 K. The best known example is dry ice, the solid form of carbon dioxide. A "magnetic analogue" of ice is also realized in some insulating magnetic materials in which the magnetic moments mimic the position of protons in water ice and obey energetic constraints similar to the Bernal-Fowlerice rules In chemistry, ice rules are basic principles that govern arrangement of atoms in water ice. They are also known as Bernal–Fowler rules, after British physicists John Desmond Bernal and Ralph H. Fowler who first described them in 1933.
The rules ...
arising from the geometrical frustration of the proton configuration in water ice. These materials are called spin ice.
See also
* * * * * * *References
External links
Webmineral listing for Ice
Estimating the bearing capacity of ice
{{Authority control Glaciology Minerals Transparent materials Articles containing video clips Limnology Oceanography Cryosphere