Hydroxyl Radicals on:
[Wikipedia]
[Google]
[Amazon]
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of
Next Generation Hybrid Photo-Catalytic Oxidation (PCO) for Trace Contaminant Control (H-PCO)
, have now made it possible to reproduce the outdoor effects of hydroxyl radicals indoors, enabling the continuous deactivation of viruses and bacteria, removal of toxic gases (such as
Engineered Water Nanostructures
(EWNS) are synthesized using two processes in parallel, namely, electrospraying and ionization of water. Pressurized water exits a hypodermic needle into an electric field (3–5 kV) to produce a large number of reactive oxygen species (ROS), primarily hydroxyl (OH•) and superoxide () radicals. Good results were reported inactivating pathogens.
Hydroxyl found in atmosphere of Venus.University lecture notes from the University of Colorado on Atmospheric Chemistry.Hydroxyl Air Purifier.
{{DEFAULTSORT:Hydroxyl Radical Alcohols Biological processes Environmental chemistry Free radicals Hydroxides Reactive intermediates Reactive oxygen species
hydroperoxides
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. O ...
(ROOH) or, in atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteoro ...
, by the reaction of excited atomic oxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
with water. It is also important in the field of radiation chemistry Radiation chemistry is a subdivision of nuclear chemistry which is the study of the chemical effects of radiation on matter; this is very different from radiochemistry as no radioactivity needs to be present in the material which is being chemically ...
, since it leads to the formation of hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, which can enhance corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
and SCC in coolant systems subjected to radioactive environments.
In organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, hydroxyl radicals are most commonly generated by photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of 1-hydroxy-2(1''H'')-pyridinethione.
Notation
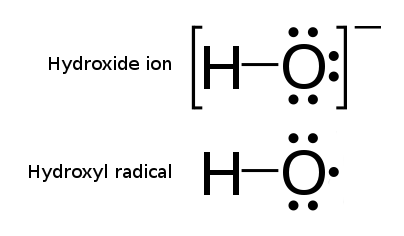
The unpaired electron of the hydroxyl radical is officially represented by amiddle dot
An interpunct , also known as an interpoint, middle dot, middot and centered dot or centred dot, is a punctuation mark consisting of a vertically centered dot used for interword separation in ancient Latin script. (Word-separating spaces did no ...
, •, beside the O.
Biology
Hydroxyl radicals can occasionally be produced as a byproduct of immune action.Macrophages
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer ce ...
and microglia
Microglia are a type of neuroglia (glial cell) located throughout the brain and spinal cord. Microglia account for about 7% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune de ...
most frequently generate this compound when exposed to very specific pathogens
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
, such as certain bacteria. The destructive action of hydroxyl radicals has been implicated in several neurological
Neurology (from el, νεῦρον (neûron), "string, nerve" and the suffix -logia, "study of") is the branch of medicine dealing with the diagnosis and treatment of all categories of conditions and disease involving the brain, the spinal ...
autoimmune diseases
An autoimmune disease is a condition arising from an abnormal immune response to a functioning body part. At least 80 types of autoimmune diseases have been identified, with some evidence suggesting that there may be more than 100 types. Nearly a ...
such as HAND
A hand is a prehensile, multi-fingered appendage located at the end of the forearm or forelimb of primates such as humans, chimpanzees, monkeys, and lemurs. A few other vertebrates such as the koala (which has two opposable thumbs on each "h ...
when immune cells become over-activated and toxic to neighboring healthy cells.
The hydroxyl radical can damage virtually all types of macromolecules: carbohydrates, nucleic acids (mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mi ...
s), lipids (lipid peroxidation
Lipid peroxidation is the chain of reactions of oxidative degradation of lipids. It is the process in which radical (chemistry), free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by ...
), and amino acids (e.g. conversion of phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino a ...
to ''m''-tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
and ''o''-tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
). The hydroxyl radical has a very short ''in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and ...
'' half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of approximately 10−9 seconds and a high reactivity. This makes it a very dangerous compound to the organism. However, humans, animals and plants have evolved to coexist with hydroxyl radicals, and hydroxyl radicals cannot enter the blood stream or tissues within the body.
Unlike superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the ...
, which can be detoxified by superoxide dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen me ...
, the hydroxyl radical cannot be eliminated by an enzymatic
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
reaction.
Effects on pathogens
Hydroxyl radicals are known to be important in the activity of some disinfectants, because they attack essential cell components in bacteria (both gram -ve and +ve) and oxidise the surface structures of viruses. Hydroxyl radicals disrupt the lipid envelope and/or capsid around the virus, causing lysing. They also penetrate the virus’s interior and disrupt the genome. These actions inactivate the virus. The disinfectant properties ofhydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
arise from these mechanisms.
Effects on allergens
Hydroxyl radicals have been shown to modify the IgE-binding capacity in pollens, spores and pet dander through the degradation and modification of the tertiary structure and/or the induction of protein denaturation and/or aggregation, resulting in a modified allergen structure. Hydroxyl radicals instantly denature Der p1 and Der f1 (house dust mites
House dust mites (HDM, or simply dust mites) are various species of acariform mites belonging to the family Pyroglyphidae that are found in association with dust in dwellings. They are known for causing allergies.
Biology
Species
The current ...
). Hydroxyl radicals oxidise their protein structures, for example causing protein backbone damage due primarily to a hydrogen abstraction or oxygen addition. Both hydroxyl radical initiated oxidation mechanisms result in a modified allergen structure. Modified allergen structures are no longer recognised by the immune system and therefore histamine and other chemical mediators are not released.
Water purification
Hydroxyl radicals play a key role in the oxidative destruction of organic pollutants using a series of methodologies collectively known asadvanced oxidation process
Advanced oxidation processes (AOPs), in a broad sense, are a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and wastewater by oxidation through reactions with hydroxyl radicals (·OH) ...
es (AOPs). The destruction of pollutants in AOPs is based on the non-selective reaction of hydroxyl radicals on organic compounds. It is highly effective against a series of pollutants including pesticides
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampric ...
, pharmaceutical compounds, dyes
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
, etc.
Air purification
The hydroxyl radical is often referred to as the "detergent" of thetroposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
because it reacts with many pollutants, decomposing them, often acting as the first step to their removal. It also has an important role in eliminating some greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
es like methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
and ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
, as well as inactivating pathogenic viruses
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsky's 1 ...
and bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
and neutralising allergenic pollens and mould spores. The rate of reaction with the hydroxyl radical often determines how long many pollutants last in the atmosphere, if they do not undergo photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
or being rained out. For instance methane, which reacts relatively slowly with hydroxyl radicals, has an average lifetime of over 5 years and many CFC
CFC, cfc, or Cfc may stand for:
Science and technology
* Chlorofluorocarbon, a class of chemical compounds
* Cardiofaciocutaneous Syndrome, a rare and serious genetic disorder
* Subpolar oceanic climate (''Cfc'' in the Köppen climate classific ...
s have lifetimes of 50 years or more. Other pollutants, such as larger hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
, can have very short average lifetimes of less than a few hours.
The first reaction with many volatile organic compounds
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a ...
(VOCs) is the removal of a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
atom, forming water and an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
radical (R•).
:•OH + RH → H2O + R•
The alkyl radical will typically react rapidly with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
forming a peroxy
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
radical.
:R• + O2 → RO
The fate of this radical in the troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
is dependent on factors such as the amount of sunlight, pollution in the atmosphere and the nature of the alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
radical that formed it.
The atmospheric chemistry leading to hydroxyl radical creation is generally absent indoors. However, new technologies, pioneered by NASA (seNext Generation Hybrid Photo-Catalytic Oxidation (PCO) for Trace Contaminant Control (H-PCO)
, have now made it possible to reproduce the outdoor effects of hydroxyl radicals indoors, enabling the continuous deactivation of viruses and bacteria, removal of toxic gases (such as
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
, carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
and formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
) and odours, and neutralisation of allergens throughout an inside space. In a similar developmentEngineered Water Nanostructures
(EWNS) are synthesized using two processes in parallel, namely, electrospraying and ionization of water. Pressurized water exits a hypodermic needle into an electric field (3–5 kV) to produce a large number of reactive oxygen species (ROS), primarily hydroxyl (OH•) and superoxide () radicals. Good results were reported inactivating pathogens.
In Earth's atmosphere
Hydroxyl radicals are created in the atmosphere by two principal chemical reactions: * During daylight hours, a photochemical reaction occurs in the atmosphere where different wavelengths of light interact with water and terpenes (secreted from plants) in the air to produce simpler by-products known asReactive Oxygen Species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
(ROS). One of the main types of ROS is the hydroxyl radical.
* In addition, during the entire 24-hour cycle, OH is formed through the reaction between terpenes and ozone.
The hydroxyl •OH radical is one of the main chemical species controlling the oxidizing capacity of the global Earth atmosphere. This oxidizing reactive species has a major impact on the concentrations and distribution of greenhouse gases and pollutants in the Earth atmosphere. It is the most widespread oxidizer in the troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
, the lowest part of the atmosphere. Understanding •OH variability is important to evaluating human impacts on the atmosphere and climate. The •OH species has a lifetime in the Earth atmosphere of less than one second. Understanding the role of •OH in the oxidation process of methane (CH4) present in the atmosphere to first carbon monoxide (CO) and then carbon dioxide (CO2) is important for assessing the residence time of this greenhouse gas, the overall carbon budget
A carbon budget is "the maximum amount of cumulative net global anthropogenic carbon dioxide () emissions that would result in limiting global warming to a given level with a given probability, taking into account the effect of other anthropogen ...
of the troposphere, and its influence on the process of global warming. The lifetime of •OH radicals in the Earth atmosphere is very short, therefore •OH concentrations in the air are very low and very sensitive techniques are required for its direct detection. Global average hydroxyl radical concentrations have been measured indirectly by analyzing methyl chloroform
The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colorless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protoc ...
(CH3CCl3) present in the air. The results obtained by Montzka ''et al.'' (2011) shows that the interannual variability in •OH estimated from CH3CCl3 measurements is small, indicating that global •OH is generally well buffered against perturbations. This small variability is consistent with measurements of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
and other trace gases primarily oxidized by •OH, as well as global photochemical model calculations.
In 2014, researchers reported their discovery of a "hole" or absence of hydroxyl throughout the entire depth of the troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
across a large region of the tropical West Pacific. They suggested that this hole is permitting large quantities of ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
-degrading chemicals to reach the stratosphere
The stratosphere () is the second layer of the atmosphere of the Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air ...
, and that this may be significantly reinforcing ozone depletion in the polar regions with potential consequences for the climate of the Earth.
Astronomy
First interstellar detection
The first experimental evidence for the presence of 18 cm absorption lines of the hydroxyl (•OH) radical in the radio absorption spectrum of Cassiopeia A was obtained by Weinreb et al. based on observations made during the period October 15–29, 1963.Important subsequent detections
Energy levels
•OH is a diatomic molecule. The electronic angular momentum along the molecular axis is +1 or −1, and the electronic spin angular momentum ''S'' = . Because of the orbit-spin coupling, the spin angular momentum can be oriented in parallel or anti parallel directions to the orbital angular momentum, producing the splitting into Π and Π states. The 2Π ground state of •OH is split by lambda doubling interaction (an interaction between the nuclei rotation and the unpaired electron motion around its orbit). Hyperfine interaction with the unpaired spin of the proton further splits the levels.Chemistry
In order to study gas phase interstellar chemistry, it is convenient to distinguish two types of interstellar clouds: diffuse clouds, with and , and dense clouds, with and density .(Hartquist, ''Molecular Astrophysics'', 1990).Production pathways
The •OH radical is linked with the production of H2O in molecular clouds. Studies of •OH distribution in Taurus Molecular Cloud-1 (TMC-1) suggest that in dense gas, •OH is mainly formed by dissociative recombination of H3O+. Dissociative recombination is the reaction in which a molecular ion recombines with an electron and dissociates into neutral fragments. Important formation mechanisms for •OH are:Destruction pathways
Small neutral molecules in the interstellar clouds may be formed by reactions of •H and •OH. The formation of O2 occurs in the gas phase via the neutral exchange reaction between O and •OH, which is also the main sink for •OH in dense regions. Atomic oxygen takes part both in the production and destruction of •OH, so the abundance of •OH depends mainly on the H3+ abundance. Then, important chemical pathways leading from •OH radicals are:Rate constants and relative rates for important formation and destruction mechanisms
Rate constants can be derived from the dataset published in a website. Rate constants have the form: : The following table has the rate constants calculated for a typical temperature in a dense cloud . : Formation rates ''r''ix can be obtained using the rate constants ''k''(''T'') and the abundances of the reactants species C and D: : where represents the abundance of the species Y. In this approach, abundances were taken from ''The UMIST database for astrochemistry 2006'', and the values are relatives to the H2 density. The following table shows the ratio in order to get a view of the most important reactions. : The results suggest that reaction is the most prominent reaction in dense clouds. It is in concordance with Harju et al. 2000. The next table shows the results by doing the same procedure for the destruction reaction: : The results show that reaction is the main sink for •OH in dense clouds.Interstellar observations
Discoveries of the microwave spectra of a considerable number of molecules prove the existence of rather complex molecules in the interstellar clouds, and provides the possibility to study dense clouds, which are obscured by the dust they contain. The •OH molecule has been observed in the interstellar medium since 1963 through its 18 cm transitions. In the subsequent years •OH was observed by its rotational transitions at far infrared wavelengths, mainly in the Orion region. Because each rotational level of •OH is split in by lambda doubling, astronomers can observe a wide variety of energy states from the ground state.Tracer of shock conditions
Very high densities are required to thermalize the rotational transitions of •OH, so it is difficult to detect far-infrared emission lines from a quiescent molecular cloud. Even at H2 densities of 106 cm−3, dust must be optically thick at infrared wavelengths. But the passage of a shock wave through a molecular cloud is precisely the process which can bring the molecular gas out of equilibrium with the dust, making observations of far-infrared emission lines possible. A moderately fast shock may produce a transient raise in the •OH abundance relative to hydrogen. So, it is possible that far-infrared emission lines of •OH can be a good diagnostic of shock conditions.In diffuse clouds
Diffuse clouds are of astronomical interest because they play a primary role in the evolution and thermodynamics of ISM. Observation of the abundant atomic hydrogen in 21 cm has shown good signal-to-noise ratio in both emission and absorption. Nevertheless, HI observations have a fundamental difficulty when they are directed at low mass regions of the hydrogen nucleus, as the center part of a diffuse cloud: the thermal width of the hydrogen lines are of the same order as the internal velocities of structures of interest, so cloud components of various temperatures and central velocities are indistinguishable in the spectrum. Molecular line observations in principle do not suffer from this problem. Unlike HI, molecules generally haveexcitation temperature
The excitation temperature (T_) is defined for a population of particles via the Boltzmann factor. It satisfies
:
\frac = \frac \exp,
where ''n''u and ''n''l represent the number of particles in an upper (''e.g.'' excited) and lower (''e.g.'' ...
''T''ex ≪ ''T''kin, so that emission is very weak even from abundant species. CO and •OH are the most easily studied candidate molecules. CO has transitions in a region of the spectrum (wavelength < 3 mm) where there are not strong background continuum sources, but •OH has the 18 cm emission, line convenient for absorption observations. Observation studies provide the most sensitive means of detections of molecules with subthermal excitation, and can give the opacity of the spectral line, which is a central issue to model the molecular region.
Studies based in the kinematic comparison of •OH and H I absorption lines from diffuse clouds are useful in determining their physical conditions, especially because heavier elements provide higher velocity resolution.
Masers
•OHmaser
A maser (, an acronym for microwave amplification by stimulated emission of radiation) is a device that produces coherent electromagnetic waves through amplification by stimulated emission. The first maser was built by Charles H. Townes, Ja ...
s, a type of astrophysical maser
An astrophysical maser is a naturally occurring source of stimulated spectral line emission, typically in the microwave portion of the electromagnetic spectrum. This emission may arise in molecular clouds, comets, planetary atmospheres, stellar at ...
, were the first masers to be discovered in space and have been observed in more environments than any other type of maser.
In the Milky Way
The Milky Way is the galaxy that includes our Solar System, with the name describing the galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars that cannot be individually distinguished by the naked eye ...
, •OH masers are found in stellar masers (evolved stars), interstellar masers (regions of massive star formation), or in the interface between supernova remnants and molecular material. Interstellar •OH masers are often observed from molecular material surrounding ultracompact H II region
An H II region or HII region is a region of interstellar atomic hydrogen that is ionized. It is typically in a molecular cloud of partially ionized gas in which star formation has recently taken place, with a size ranging from one to hundred ...
s (UC H II). But there are masers associated with very young stars that have yet to create UC H II regions. This class of •OH masers appears to form near the edges of very dense material, place where H2O masers form, and where total densities drop rapidly and UV radiation form young stars can dissociate the H2O molecules. So, observations of •OH masers in these regions, can be an important way to probe the distribution of the important H2O molecule in interstellar shocks at high spatial resolutions.
See also
*Hydroxyl ion absorption Hydroxyl ion absorption is the absorption in optical fibers of electromagnetic waves, including the near-infrared, due to the presence of trapped hydroxyl ions remaining from water as a contaminant.
The hydroxyl (OH−) ion, can penetrate glass du ...
*Hydrogen darkening
Hydrogen darkening is a physical degradation of the optical properties of glass. Free hydrogen atoms are able to bind to the SiO2 silica glass compound forming hydroxyl (OH)—a chemical compound that interferes with the passage of light through ...
*Hydrogen cycle
The hydrogen cycle consists of hydrogen exchanges between biotic (living) and abiotic (non-living) sources and sinks of hydrogen-containing compounds.
Hydrogen (H) is the most abundant element in the universe. On Earth, common H-containing inorg ...
References
*External links
Hydroxyl found in atmosphere of Venus.
{{DEFAULTSORT:Hydroxyl Radical Alcohols Biological processes Environmental chemistry Free radicals Hydroxides Reactive intermediates Reactive oxygen species