Host Defense Peptide on:
[Wikipedia]
[Google]
[Amazon]
 Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the
 Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are generally between 12 and 50 amino acids. These peptides include two or more positively charged residues provided by
Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are generally between 12 and 50 amino acids. These peptides include two or more positively charged residues provided by
 The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The cytoplasmic membrane is a frequent target, but peptides may also interfere with DNA and
The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The cytoplasmic membrane is a frequent target, but peptides may also interfere with DNA and
 Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular, solid-state NMR studies have provided an atomic-level resolution explanation of membrane disruption by antimicrobial peptides. In more recent years,
Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular, solid-state NMR studies have provided an atomic-level resolution explanation of membrane disruption by antimicrobial peptides. In more recent years,
and LAMP (Linking AMPs). The Antimicrobial peptide databases may be divided into two categories on the basis of the source of peptides it contains, as specific databases and general databases. These databases have various tools for antimicrobial peptides analysis and prediction. For example, the APD has a widely used calculation interface. It also provides links to many other tools. CAMP contains AMP prediction, feature calculator, BLAST search, ClustalW, VAST, PRATT, Helical wheel etc. In addition, ADAM allows users to search or browse through AMP sequence-structure relationships. Antimicrobial peptides often encompass a wide range of categories such as antifungal, antibacterial, and antituberculosis peptides. : Provides an online platform for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. is an online resource that addresses various topics such as annotations of antimicrobial peptides (AMPs) including sequence information, antimicrobial activities, post-translational modifications (PTMs), structural visualization, antimicrobial potency, target species with minimum inhibitory concentration (MIC), physicochemical properties, or AMP–protein interactions. Tools such as PeptideRanker, PeptideLocator, and AntiMPmod allow for the prediction of antimicrobial peptides while others have been developed to predict antifungal and anti-Tuberculosis activities.
ADAM (A Database of Anti-Microbial peptides)
at ntou.edu.tw
AntiFP
Prediction of antifungal peptides
AntiMPmod
Prediction of antimicrobial potential of modified peptides *
AntiTbPred
Prediction of anti-tuberculosis peptides
Antimicrobial Peptide Database
at University of Nebraska Medical Center
Antimicrobial Peptide Scanner
Deep Learning based AMP prediction server
AntiTbPdb
Anti Tubercular Peptide Database
BioPD
at Peking University Health Science Center
CAMP:Collection of Anti-Microbial Peptides
at National Institute for Research in Reproductive Health (NIRRH)
DBAASP
- Database of Antimicrobial Activity and Structure of Peptides]
LAMP
at Fudan University
PeptideLocator
Prediction of functional peptides, including antimicrobial peptides, in a protein sequence
PeptideRanker
Bioactive peptide, including antimicrobial peptide, prediction
modlAMP
Python package for computational work with antimicrobial peptides, including sequence handling, -design, -prediction, descriptor calculation and plotting {{DEFAULTSORT:Antimicrobial Peptides Antimicrobial peptides, Immunology Immune system Peripheral membrane proteins Insect immunity
 Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response
The innate, or nonspecific, immune system is one of the two main immunity strategies (the other being the adaptive immune system) in vertebrates. The innate immune system is an older evolutionary defense strategy, relatively speaking, and is the ...
found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
cells that may represent targets for antimicrobial peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
s. These peptides are potent, broad spectrum antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
s which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
Gram-positive bact ...
bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membrane
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell from the external environment or creates intracellular compartments by serving as a boundary between one part of the ce ...
s, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.
Structure
arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the am ...
, lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −C ...
or, in acidic environments, histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the de ...
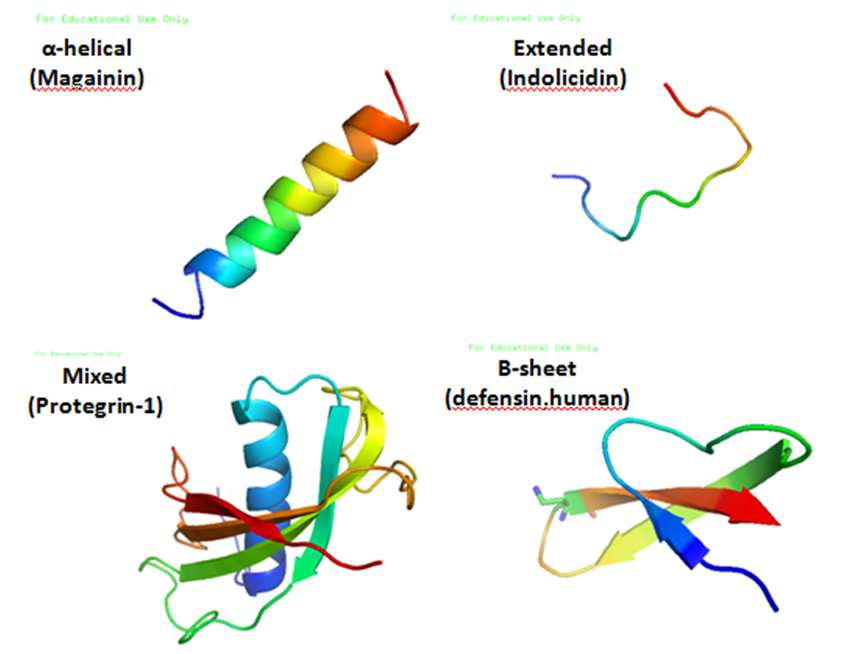
, and a large proportion (generally >50%) of hydrophobic residues. The secondary structures of these molecules follow 4 themes, including i) α-helical, ii) β-stranded due to the presence of 2 or more disulfide bonds, iii) β-hairpin or loop due to the presence of a single disulfide bond and/or cyclization of the peptide chain, and iv) extended. Many of these peptides are unstructured in free solution, and fold into their final configuration upon partitioning into biological membranes. It contains hydrophilic amino acid residues aligned along one side and hydrophobic amino acid residues aligned along the opposite side of a helical molecule. This amphipathicity of the antimicrobial peptides allows them to partition into the membrane lipid bilayer. The ability to associate with membranes is a definitive feature of antimicrobial peptides, although membrane permeabilization is not necessary. These peptides have a variety of antimicrobial activities ranging from membrane permeabilization to action on a range of cytoplasmic targets.
Activities
 The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The cytoplasmic membrane is a frequent target, but peptides may also interfere with DNA and
The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The cytoplasmic membrane is a frequent target, but peptides may also interfere with DNA and protein synthesis
Protein biosynthesis (or protein synthesis) is a core biological process, occurring inside Cell (biology), cells, homeostasis, balancing the loss of cellular proteins (via Proteolysis, degradation or Protein targeting, export) through the product ...
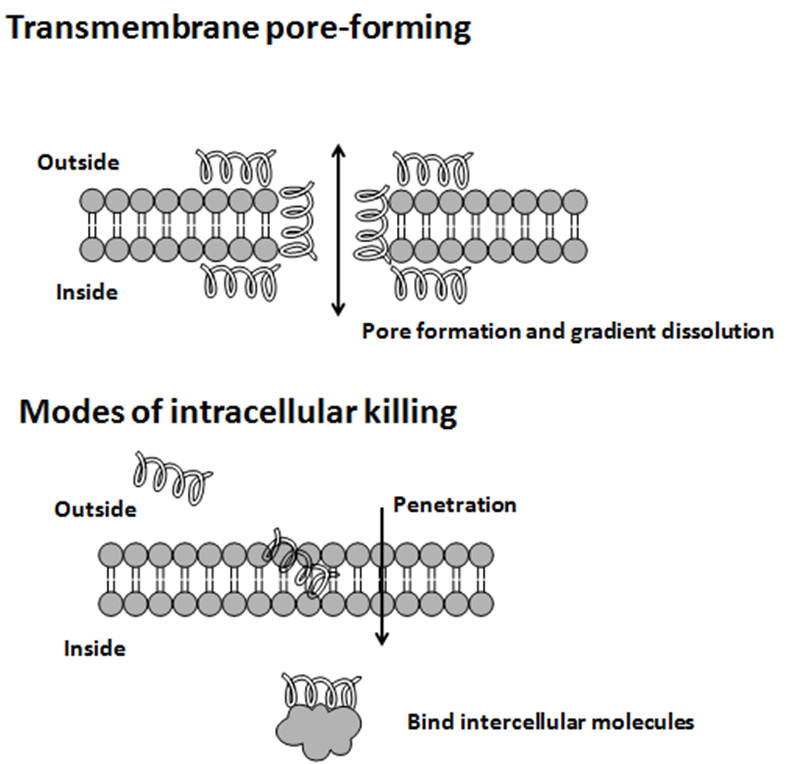
, protein folding, and cell wall synthesis. The initial contact between the peptide and the target organism is electrostatic, as most bacterial surfaces are anionic, or hydrophobic, such as in the antimicrobial peptide Piscidin. Their amino acid composition, amphipathicity, cationic charge and size allow them to attach to and insert into membrane bilayers to form pores by ‘barrel-stave’, ‘carpet’ or ‘toroidal-pore’ mechanisms. Alternately, they may penetrate into the cell to bind intracellular molecules which are crucial to cell living.
Intracellular binding models includes inhibition of cell wall synthesis, alteration of the cytoplasmic membrane, activation of autolysin, inhibition of DNA, RNA, and protein synthesis, and inhibition of certain enzymes. However, in many cases, the exact mechanism of killing is not known. One emerging technique for the study of such mechanisms is dual polarisation interferometry
Dual-polarization interferometry (DPI) is an analytical technique that probes molecular layers adsorbed to the surface of a waveguide using the evanescent wave of a laser beam. It is used to measure the conformational change in proteins, or othe ...
. In contrast to many conventional antibiotics these peptides appear to be bactericidal
A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance which kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics.
However, material surfaces can also have bactericidal properties based solely on their ...
instead of bacteriostatic. In general the antimicrobial activity of these peptides is determined by measuring the minimal inhibitory concentration (MIC), which is the lowest concentration of drug that inhibits bacterial growth.
AMPs can possess multiple activities including anti-gram-positive bacterial, anti-gram-negative bacterial, anti-fungal, anti-viral, anti-parasitic, and anti cancer activities. A big AMP functional analysis indicates that among all AMP activities, amphipathicity and charge, two major properties of AMPs, best distinguish between AMPs with and without anti-gram-negative bacterial activities. This implies that being AMPs with anti-gram-negative bacterial activities may prefer or even require strong amphipathicity and net positive charge.
Immunomodulation
In addition to killing bacteria directly they have been demonstrated to have a number of immunomodulatory functions that may be involved in the clearance of infection, including the ability to alter host gene expression, act as chemokines and/or inducechemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In additio ...
production, inhibiting lipopolysaccharide
Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide that are bacterial toxins. They are composed of an O-antigen, an outer core, and an inner core all joined by a covalent bond, and are found in the outer m ...
induced pro-inflammatory cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
production, promoting wound healing, and modulating the responses of dendritic cell
Dendritic cells (DCs) are antigen-presenting cells (also known as ''accessory cells'') of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to the T cells of the immune system. ...
s and cells of the adaptive immune response. Animal models indicate that host defense peptides are crucial for both prevention and clearance of infection. It appears as though many peptides initially isolated as and termed "antimicrobial peptides" have been shown to have more significant alternative functions in vivo (e.g. hepcidin
). Dusquetide for example is an immunomodulator that acts through p62, a protein involved in toll like receptor based signalling of infection. The peptide is being examined in a Phase III clinical trial by Soligenix (SGNX) to ascertain if it can assist in repair of radiation-induced damage to oral mucosa arising during cancer radiotherapy of the head and neck.
Mechanisms of action
 Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular, solid-state NMR studies have provided an atomic-level resolution explanation of membrane disruption by antimicrobial peptides. In more recent years,
Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular, solid-state NMR studies have provided an atomic-level resolution explanation of membrane disruption by antimicrobial peptides. In more recent years, X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
has been used to delineate in atomic detail how the family of plant defensins rupture membranes by identifying key phospholipids in the cell membranes of the pathogen. Human defensins have been thought to act through a similar mechanism, targeting cell membrane lipids as part of their function. In fact human beta-defensin 2
Beta-defensin 2 (BD-2) also known as skin-antimicrobial peptide 1 (SAP1) is a peptide that in humans is encoded by the ''DEFB4'' (defensin, beta 4) gene.
Human beta-defensin-2 (hBD-2) is a cysteine-rich cationic low molecular weight antimicrobial ...
have now been shown to kill the pathogenic fungi '' Candida albicans'' through interactions with specific phospholipids. From the computational point of view, the molecular dynamics simulations can shed light in the molecular mechanism and the specific peptide interactions with lipids, ions and solvent.
Therapeutic research and use
Antimicrobial peptides have been used as therapeutic agents; their use is generally limited to intravenous administration or topical applications due to their short half-lives. As of January 2018 the following antimicrobial peptides were in clinical use: *Bacitracin
Bacitracin is a polypeptide antibiotic. It is a mixture of related cyclic peptides produced by ''Bacillus licheniformis'' bacteria, that was first isolated from the variety "Tracy I" ( ATCC 10716) in 1945. These peptides disrupt Gram-positive bac ...
for pneumonia, topical
* Boceprevir
Boceprevir (INN, trade name Victrelis) is a protease inhibitor used to treat hepatitis caused by hepatitis C virus (HCV) genotype 1. It binds to the HCV nonstructural protein 3 active site.
It was initially developed by Schering-Plough, then b ...
, Hepatitis C (oral, cyclic peptide)
* Dalbavancin
Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatm ...
, bacterial infections, IV
* Daptomycin, bacterial infections, IV
* Enfuvirtide, HIV, subcutaneous injection
* Oritavancin
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vanc ...
, bacterial infections, IV
* Teicoplanin
Teicoplanin is an antibiotic used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant ''Staphylococcus aureus'' and ''Enterococcus faecalis''. It is a semisynthetic glycopeptide ...
, bacterial infections, IV
* Telaprevir, Hepatitis C, oral cyclic peptide
* Telavancin
Telavancin (trade name Vibativ) is a bactericidal lipoglycopeptide for use in MRSA or other Gram-positive infections. Telavancin is a semi-synthetic derivative of vancomycin.
The FDA approved the drug in September 2009 for complicated skin and ...
, bacterial infection, IV
* Vancomycin
Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is recommended intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, ...
, bacterial infection, IV.
* Guavanin 2, bacterial infection against Gram-positive and Gram-negative also.
Activity beyond antibacterial functions
AMPs have been observed having functions other than bacterial and fungal killing. These activities include antiviral effects, but also roles in host defence such as anticancer functions and roles in neurology. This has led to a movement for re-branding AMPs as "Host-defence peptides" to encompass the broad scope of activities AMPs can have.Anticancer properties
Some cecropins (e.g. cecropin A, and cecropin B) have anticancer properties and are called anticancer peptides (ACPs). Hybrid ACPs based on Cecropin A have been studied for anticancer properties. The fruit fly Defensin prevents tumour growth, suspected to bind to tumour cells owing to cell membrane modifications common to most cancer cells, such as phosphatidylserine exposure.Antibiofilm properties
Cecropin A can destroy planktonic and sessilebiofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular ...
-forming uropathogenic ''E. coli'' (UPEC) cells, either alone or when combined with the antibiotic nalidixic acid, synergistically clearing infection in vivo (in the insect host '' Galleria mellonella'') without off-target cytotoxicity. The multi-target mechanism of action involves outer membrane permeabilization followed by biofilm disruption triggered by the inhibition of efflux pump activity and interactions with extracellular and intracellular nucleic acids.
Other research
Recently there has been some research to identify potential antimicrobial peptides from prokaryotes, aquatic organisms such as fish, and shellfish, andmonotreme
Monotremes () are prototherian mammals of the order Monotremata. They are one of the three groups of living mammals, along with placentals (Eutheria), and marsupials (Metatheria). Monotremes are typified by structural differences in their brain ...
s such as echidnas.
Selectivity
In the competition of bacterial cells and host cells with the antimicrobial peptides, antimicrobial peptides will preferentially interact with the bacterial cell to the mammalian cells, which enables them to kill microorganisms without being significantly toxic to mammalian cells. Selectivity is a very important feature of the antimicrobial peptides and it can guarantee their function as antibiotics in host defense systems. With regard tocancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
cells, they themselves also secrete human antimicrobial peptides including defensin
Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () ( chordates with backbones), including all mammals, birds, reptiles, ...
, and in some cases, they are reported to be more resistant than the surrounding normal cells.
Therefore, we cannot conclude that selectivity is always high against cancer cells.
Factors
There are some factors that are closely related to the selectivity property of antimicrobial peptides, among which the cationic property contributes most. Since the surface of the bacterial membranes is more negatively charged than mammalian cells, antimicrobial peptides will show different affinities towards the bacterial membranes and mammalian cell membranes. In addition, there are also other factors that will affect the selectivity. It's well known thatcholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell mem ...
is normally widely distributed in the mammalian cell membranes as a membrane stabilizing agents but absent in bacterial cell membranes; and the presence of these cholesterols will also generally reduce the activities of the antimicrobial peptides, due either to stabilization of the lipid bilayer or to interactions between cholesterol and the peptide. So the cholesterol in mammalian cells will protect the cells from attack by the antimicrobial peptides.
Besides, the transmembrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges ...
is well known to affect peptide-lipid interactions. There's an inside-negative transmembrane potential existing from the outer leaflet to the inner leaflet of the cell membranes and this inside-negative transmembrane potential will facilitate membrane permeabilization probably by facilitating the insertion of positively charged peptides into membranes. By comparison, the transmembrane potential of bacterial cells is more negative than that of normal mammalian cells, so bacterial membrane will be prone to be attacked by the positively charged antimicrobial peptides.
Similarly, it is also believed that increasing ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as ...
, which in general reduces the activity of most antimicrobial peptides, contributes partially to the selectivity of the antimicrobial peptides by weakening the electrostatic interactions required for the initial interaction.
Mechanism
The cell membranes of bacteria are rich in acidicphospholipids
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
, such as phosphatidylglycerol
Phosphatidylglycerol is a glycerophospholipid found in pulmonary surfactant and in the plasma membrane where it directly activates lipid-gated ion channels.
The general structure of phosphatidylglycerol consists of a L-glycerol 3-phosphate backbo ...
and cardiolipin
Cardiolipin (IUPAC name 1,3-bis(''sn''-3’-phosphatidyl)-''sn''-glycerol) is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition. It can also be found in the membranes of most ...
. These phospholipid headgroups are heavily negatively charged. Therefore, the outmost leaflets of the bilayer which is exposed to the outside of the bacterial membranes are more attractive to the attack of the positively charged antimicrobial peptides. So the interaction between the positive charges of antimicrobial peptides and the negatively charged bacterial membranes is mainly the electrostatic interactions, which is the major driving force for cellular association. In addition, since antimicrobial peptides form structures with a positively charged face as well as a hydrophobic face, there are also some hydrophobic interactions between the hydrophobic regions of the antimicrobial peptides and the zwitterionic phospholipids (electrically neutral) surface of the bacterial membranes, which act only as a minor effect in this case.
In contrast, the outer part of the membranes of plants and mammals is mainly composed of lipids without any net charges since most of the lipids with negatively charged headgroups are principally sequestered into the inner leaflet of the plasma membranes. Thus in the case of mammalian cells, the outer surfaces of the membranes are usually made of zwitterionic phosphatidylcholine and sphingomyelin
Sphingomyelin (SPH, ˌsfɪŋɡoˈmaɪəlɪn) is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a ethano ...
, even though a small portion of the membrane's outer surfaces contain some negatively charged gangliosides. Therefore, the hydrophobic interaction between the hydrophobic face of amphipathic antimicrobial peptides and the zwitterionic phospholipids on the cell surface of mammalian cell membranes plays a major role in the formation of peptide-cell binding. However, the hydrophobic interaction is relatively weak when compared to the electrostatic interaction, thus, the antimicrobial peptides will preferentially interact with bacterial membranes.
Dual polarisation interferometry
Dual-polarization interferometry (DPI) is an analytical technique that probes molecular layers adsorbed to the surface of a waveguide using the evanescent wave of a laser beam. It is used to measure the conformational change in proteins, or othe ...
has been used ''in vitro'' to study and quantify the association to headgroup, insertion into the bilayer, pore formation and eventual disruption of the membrane.
Control
A lot of effort has been put into controlling cell selectivity. For example, attempts have been made to modify and optimize the physicochemical parameters of the peptides to control the selectivities, including net charge, helicity, hydrophobicity per residue (H), hydrophobic moment (μ) and the angle subtended by the positively charged polar helix face (Φ). Other mechanisms like the introduction of D-amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
and fluorinated amino acids in the hydrophobic phase are believed to break the secondary structure and thus reduce hydrophobic interaction with mammalian cells. It has also been found that Pro→Nlys substitution in Pro-containing β-turn antimicrobial peptides was a promising strategy for the design of new small bacterial cell-selective antimicrobial peptides with intracellular mechanisms of action. It has been suggested that direct attachment of magainin to the substrate surface decreased nonspecific cell binding and led to improved detection limit for bacterial cells such as ''Salmonella
''Salmonella'' is a genus of rod-shaped (bacillus) Gram-negative bacteria of the family Enterobacteriaceae. The two species of ''Salmonella'' are ''Salmonella enterica'' and ''Salmonella bongori''. ''S. enterica'' is the type species and is fur ...
'' and ''E. coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
''.
Bacterial resistance
Bacteria use various resistance strategies to avoid antimicrobial peptide killing. Some microorganisms alter net surface charges. ''Staphylococcus aureus
''Staphylococcus aureus'' is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive ...
'' transports D-alanine from the cytoplasm to the surface teichoic acid which reduces the net negative charge by introducing basic amino groups. ''S. aureus'' also modifies its anionic membranes via MprF with L-lysine, increasing the positive net charge. The interaction of antimicrobial peptides with membrane targets can be limited by capsule polysaccharide of ''Klebsiella pneumoniae''.
Alterations occur in Lipid A. ''Salmonella'' species reduce the fluidity of their outer membrane by increasing hydrophobic interactions between an increased number of Lipid A acyl tails by adding myristate to Lipid A with 2-hydroxymyristate and forming hepta-acylated Lipid A by adding palmitate. The increased hydrophobic moment is thought to retard or abolish antimicrobial peptide insertion and pore formation. The residues undergo alteration in membrane proteins. In some Gram-negative bacteria, alteration in the production of outer membrane proteins correlates with resistance to killing by antimicrobial peptides. Non-typeable '' Hemophilus influenzae'' transports AMPs into the interior of the cell, where they are degraded. Furthermore, ''H. influenzae'' remodels its membranes to make it appear as if the bacterium has already been successfully attacked by AMPs, protecting it from being attacked by more AMPs. ATP-binding cassette transporters import antimicrobial peptides and the resistance-nodulation cell-division efflux pump exports antimicrobial peptides. Both transporters have been associated with antimicrobial peptide resistance. Bacteria produce proteolytic enzymes, which may degrade antimicrobial peptides leading to their resistance. Outer membrane vesicles produced by Gram-negative bacteria bind the antimicrobial peptides and sequester them away from the cells, thereby protecting the cells. The outer membrane vesicles are also known to contain various proteases, peptidases and other lytic enzymes, which may have a role in degrading the extracellular peptide and nucleic acid molecules, which if allowed to reach to the bacterial cells may be dangerous for the cells. Cyclic-di-GMP signaling had also been involved in the regulation of antimicrobial peptide resistance in ''Pseudomonas aeruginosa
''Pseudomonas aeruginosa'' is a common encapsulated, gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, ''P. aerugi ...
''
While these examples show that resistance can evolve naturally, there is increasing concern that using pharmaceutical copies of antimicrobial peptides can make resistance happen more often and faster. In some cases, resistance to these peptides used as a pharmaceutical to treat medical problems can lead to resistance, not only to the medical application of the peptides, but to the physiological function of those peptides.
The ‘Trojan Horse’ approach to solving this problem capitalizes on the innate need for iron by pathogens. “Smuggling” antimicrobials into the pathogen is accomplished by linking them to siderophores for transport. While simple in concept, it has taken many decades of work to accomplish the difficult hurdle of transporting antimicrobials across the cell membranes of pathogens. Lessons learned from the successes and failures of siderophore-conjugate drugs evaluated during the development of novel agents using the ‘Trojan horse’ approach have been reviewed.
Examples
Antimicrobial peptides are produced by species across the tree of life, including: * bacteria (''e.g.'' bacteriocin, and many others) * fungi (''e.g.''peptaibols
Peptaibols are biologically active peptides containing between seven and twenty amino acid residues, some of which are non-proteinogenic amino acids. In particular, they contain α-aminoisobutyric acid along with other unusual aminoacids such as e ...
, plectasin
Plectasin is an antibiotic protein from the mushroom '' Pseudoplectania nigrella''. It was initially discovered in 2005 and commercialised by Novozymes. Plectasin belongs to the antimicrobial peptide class called fungal defensins, which is also p ...
, and many others)
* cnidaria (''e.g.'' hydramacin, aurelin)
* many from insects and arthropods (''e.g.'' cecropin
Cecropins are antimicrobial peptides. They were first isolated from the hemolymph of ''Hyalophora cecropia'', whence the term cecropin was derived. Cecropins lyse bacterial cell membranes; they also inhibit proline uptake and cause leaky membranes ...
, attacin, melittin, mastoparan
Mastoparan is a peptide toxin from wasp venom. It has the chemical structure Ile-Asn-Leu-Lys-Ala-Leu-Ala-Ala-Leu-Ala-Lys-Lys-Ile-Leu-NH2.
The net effect of mastoparan's mode of action depends on cell type, but seemingly always involves exocytosis ...
, drosomycin
Drosomycin is an antifungal peptide from ''Drosophila melanogaster'' and was the first antifungal peptide isolated from insects. Drosomycin is induced by infection by the Toll signalling pathway, while expression in surface epithelia like the re ...
)
* amphibia, frogs ( magainin, dermaseptin, aurein
This family of antibacterial peptides are secreted from the granular dorsal glands of ''Litoria aurea'' (Green and golden bell frog), '' Litoria raniformis'' (Southern bell frog), '' Litoria citropa'' (Australian blue mountains tree frog) and frogs ...
, and others)
* birds (''e.g.'' avian defensins)
* and mammals (''e.g.'' cathelicidins, alpha- and beta- defensins, regIII peptides)
Research has increased in recent years to develop artificially-engineered mimics of antimicrobial peptides such as SNAPPs, in part due to the prohibitive cost of producing naturally-derived AMPs. An example of this is the facially cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic peptide C18G, which was designed from the C-terminal domain of human platelet factor IV. Currently, the most widely used antimicrobial peptide is nisin; being the only FDA
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food s ...
approved antimicrobial peptide, it is commonly used as an artificial preservative.
Bioinformatics
Several bioinformatic databases exist to catalogue antimicrobial peptides. The APD (the Antimicrobial Peptide Database) is the original and model database for antimicrobial peptides (https://aps.unmc.edu). Based on the APD, other databases have also been built, including ADAM (A Database of Anti-Microbial peptides), BioPD (Biologically active Peptide Database), CAMP (Collection of sequences and structures of antimicrobial peptides), DBAASP (Database of Antimicrobial Activity and Structure of Peptides), DRAMP(Data Repository of Antimicrobial Peptideand LAMP (Linking AMPs). The Antimicrobial peptide databases may be divided into two categories on the basis of the source of peptides it contains, as specific databases and general databases. These databases have various tools for antimicrobial peptides analysis and prediction. For example, the APD has a widely used calculation interface. It also provides links to many other tools. CAMP contains AMP prediction, feature calculator, BLAST search, ClustalW, VAST, PRATT, Helical wheel etc. In addition, ADAM allows users to search or browse through AMP sequence-structure relationships. Antimicrobial peptides often encompass a wide range of categories such as antifungal, antibacterial, and antituberculosis peptides. : Provides an online platform for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. is an online resource that addresses various topics such as annotations of antimicrobial peptides (AMPs) including sequence information, antimicrobial activities, post-translational modifications (PTMs), structural visualization, antimicrobial potency, target species with minimum inhibitory concentration (MIC), physicochemical properties, or AMP–protein interactions. Tools such as PeptideRanker, PeptideLocator, and AntiMPmod allow for the prediction of antimicrobial peptides while others have been developed to predict antifungal and anti-Tuberculosis activities.
See also
*Aurein
This family of antibacterial peptides are secreted from the granular dorsal glands of ''Litoria aurea'' (Green and golden bell frog), '' Litoria raniformis'' (Southern bell frog), '' Litoria citropa'' (Australian blue mountains tree frog) and frogs ...
* Bacteriocin
* Cathelicidin
* Copsin Copsin is a fungal defensin that acts as an antimicrobial polypeptide secreted from the inky cap mushroom, first reported at the end of 2014. The fungal defensin acts against gram positive bacteria.
History
In October 2014, a collaboration of t ...
* Diptericin
* Peripheral membrane proteins
* Virtual colony count
References
External links
ADAM (A Database of Anti-Microbial peptides)
at ntou.edu.tw
AntiFP
Prediction of antifungal peptides
AntiMPmod
Prediction of antimicrobial potential of modified peptides *
AntiTbPred
Prediction of anti-tuberculosis peptides
Antimicrobial Peptide Database
at University of Nebraska Medical Center
Antimicrobial Peptide Scanner
Deep Learning based AMP prediction server
AntiTbPdb
Anti Tubercular Peptide Database
BioPD
at Peking University Health Science Center
CAMP:Collection of Anti-Microbial Peptides
at National Institute for Research in Reproductive Health (NIRRH)
DBAASP
- Database of Antimicrobial Activity and Structure of Peptides]
LAMP
at Fudan University
PeptideLocator
Prediction of functional peptides, including antimicrobial peptides, in a protein sequence
PeptideRanker
Bioactive peptide, including antimicrobial peptide, prediction
modlAMP
Python package for computational work with antimicrobial peptides, including sequence handling, -design, -prediction, descriptor calculation and plotting {{DEFAULTSORT:Antimicrobial Peptides Antimicrobial peptides, Immunology Immune system Peripheral membrane proteins Insect immunity