Half-equation on:
[Wikipedia]
[Google]
[Amazon]
 Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of
 Oxygen is the quintessential oxidizer.
Oxygen is the quintessential oxidizer.
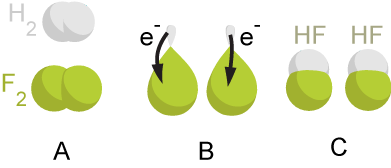 In the reaction between hydrogen and
In the reaction between hydrogen and
 In this type of reaction, a metal atom in a compound (or in a solution) is replaced by an atom of another metal. For example, copper is deposited when zinc metal is placed in a copper(II) sulfate solution:
:
In the above reaction, zinc metal displaces the copper(II) ion from copper sulfate solution and thus liberates free copper metal. The reaction is spontaneous and releases 213 kJ per 65 g of zinc.
The ionic equation for this reaction is:
:
As two half-reactions, it is seen that the zinc is oxidized:
:
And the copper is reduced:
:
In this type of reaction, a metal atom in a compound (or in a solution) is replaced by an atom of another metal. For example, copper is deposited when zinc metal is placed in a copper(II) sulfate solution:
:
In the above reaction, zinc metal displaces the copper(II) ion from copper sulfate solution and thus liberates free copper metal. The reaction is spontaneous and releases 213 kJ per 65 g of zinc.
The ionic equation for this reaction is:
:
As two half-reactions, it is seen that the zinc is oxidized:
:
And the copper is reduced:
:
 * The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of
* The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of
 Many important
Many important
 Minerals are generally oxidized derivatives of metals. Iron is mined as its magnetite (Fe3O4). Titanium is mined as its dioxide, usually in the form of rutile (TiO2). To obtain the corresponding metals, these oxides must be reduced, which is often achieved by heating these oxides with carbon or carbon monoxide as reducing agents.
Minerals are generally oxidized derivatives of metals. Iron is mined as its magnetite (Fe3O4). Titanium is mined as its dioxide, usually in the form of rutile (TiO2). To obtain the corresponding metals, these oxides must be reduced, which is often achieved by heating these oxides with carbon or carbon monoxide as reducing agents.
Chemical Equation Balancer
– An open-source chemical equation balancer that handles redox reactions.
Online redox reaction equation balancer, balances equations of any half-cell and full reactions
{{Authority control Soil chemistry Chemical reactions Articles containing video clips Redox Reaction mechanisms
 Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
There are two classes of redox reactions:
* ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials.
* ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds are reduced by transfer of hydrogen atoms.
Terminology
"Redox" is a combination of the words "reduction" and "oxidation". The term "redox" was first used in 1928. The processes of oxidation and reduction occur simultaneously and cannot occur independently. In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or ''reducing agent'' loses electrons and is oxidized, and the oxidant or ''oxidizing agent'' gains electrons and is reduced. The pair of an oxidizing and reducing agent that is involved in a particular reaction is called a ''redox pair''. A ''redox couple'' is a reducing species and its corresponding oxidizing form, e.g., / .The oxidation alone and the reduction alone are each called a '' half-reaction'' because two half-reactions always occur together to form a whole reaction.Oxidants
''Oxidation'' originally implied a reaction with oxygen to form an oxide. Later, the term was expanded to encompass oxygen-like substances that accomplished parallel chemical reactions. Ultimately, the meaning was generalized to include all processes involving the loss of electrons. Substances that have the ability to ''oxidize'' other substances (cause them to lose electrons) are said to be ''oxidative'' or ''oxidizing'', and are known as oxidizing agents, oxidants, or oxidizers. The oxidant (oxidizing agent) removes electrons from another substance, and is thus itself reduced. And, because it "accepts" electrons, the oxidizing agent is also called anelectron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
. Oxidants are usually chemical substances with elements in high oxidation states (e.g., , , , , ), or else highly electronegative elements (e.g. O2, F2, Cl2, Br2, I2) that can gain extra electrons by oxidizing another substance.
Oxidizers are oxidants, but the term is mainly reserved for sources of oxygen, particularly in the context of explosions. Nitric acid is an oxidizer.
Reducers
Substances that have the ability to ''reduce'' other substances (cause them to gain electrons) are said to be ''reductive'' or ''reducing'' and are known as reducing agents, reductants, or reducers. The reductant (reducing agent) transfers electrons to another substance and is thus itself oxidized. And, because it donates electrons, the reducing agent is also called an electron donor. Electron donors can also form charge transfer complexes with electron acceptors. The word ''reduction'' originally referred to the loss in weight upon heating a metallic ore such as a metal oxide to extract the metal. In other words, ore was "reduced" to metal. Antoine Lavoisier demonstrated that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of ''reduction'' then became generalized to include all processes involving a gain of electrons. Reducing equivalent refers to chemical species which transfer the equivalent of one electron in redox reactions. The term is common in biochemistry. A reducing equivalent can be an electron, a hydrogen atom, as ahydride ion
The hydrogen anion, H−, is a negative ion of hydrogen, that is, a hydrogen atom that has captured an extra electron. The hydrogen anion is an important constituent of the atmosphere of stars, such as the Sun. In chemistry, this ion is calle ...
.
Reductants in chemistry are very diverse. Electropositive elemental metals, such as lithium, sodium, magnesium, iron, zinc, and aluminium, are good reducing agents. These metals donate or ''give away'' electrons relatively readily. They transfer electrons.
''Hydride transfer reagents'', such as NaBH4 and LiAlH4, reduce by atom transfer: they transfer the equivalent of hydride or H−. These reagents widely used in the reduction of carbonyl compounds to alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
. A related method of reduction involves the use of hydrogen gas (H2) as sources of H atoms.
Electronation and deelectronation
The electrochemist John Bockris proposed the words ''electronation'' and ''deelectronation'' to describe reduction and oxidation processes, respectively, when they occur at electrodes. These words are analogous to protonation and deprotonation. They have not been widely adopted by chemists worldwide, although IUPAC has recognized the term electronation.Rates, mechanisms, and energies
Redox reactions can occur slowly, as in the formation of rust, or rapidly, as in the case of burning fuel. Electron transfer reactions are generally fast, occurring within the time of mixing. The mechanisms of atom-transfer reactions are highly variable because many kinds of atoms can be transferred. Such reactions can also be quite complex, i.e. involve many steps. The mechanisms of electron-transfer reactions occur by two distinct pathways, inner sphere electron transfer andouter sphere electron transfer
Outer sphere refers to an electron transfer (ET) event that occurs between chemical species that remain separate and intact before, during, and after the ET event. In contrast, for inner sphere electron transfer the participating redox sites underg ...
.
Analysis of bond energies and ionization energies in water allow calculation of the thermodynamic aspects of redox reactions.
Standard electrode potentials (reduction potentials)
Each half-reaction has a ''standard electrode potential'' (''E''), which is equal to the potential difference or voltage at equilibrium under standard conditions of an electrochemical cell in which the cathode reaction is the half-reaction considered, and the anode is a standard hydrogen electrode where hydrogen is oxidized: : H2 → H+ + e− The electrode potential of each half-reaction is also known as its ''reduction potential'' ''E'', or potential when the half-reaction takes place at a cathode. The reduction potential is a measure of the tendency of the oxidizing agent to be reduced. Its value is zero for H+ + e− → H2 by definition, positive for oxidizing agents stronger than H+ (e.g., +2.866 V for F2) and negative for oxidizing agents that are weaker than H+ (e.g., −0.763 V for Zn2+). For a redox reaction that takes place in a cell, the potential difference is: :''E'' = ''E'' – ''E'' However, the potential of the reaction at the anode is sometimes expressed as an ''oxidation potential'': :''E'' = –''E'' The oxidation potential is a measure of the tendency of the reducing agent to be oxidized but does not represent the physical potential at an electrode. With this notation, the cell voltage equation is written with a plus sign :''E'' = ''E'' + ''E''Examples of redox reactions
 In the reaction between hydrogen and
In the reaction between hydrogen and fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, hydrogen is being oxidized and fluorine is being reduced:
:
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the F-F bond. This reaction can be analyzed as two half-reactions. The oxidation reaction converts hydrogen to protons:
:
The reduction reaction converts fluorine to the fluoride anion:
:
The half reactions are combined so that the electrons cancel:
:
The protons and fluoride combine to form hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
in a non-redox reaction:
:2 H+ + 2 F− → 2 HF
The overall reaction is:
:
Metal displacement
Other examples
* The reduction ofnitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
to nitrogen in the presence of an acid ( denitrification):
::
* The combustion of hydrocarbons, such as in an internal combustion engine, produces water, carbon dioxide, some partially oxidized forms such as carbon monoxide, and heat energy. Complete oxidation of materials containing carbon produces carbon dioxide.
* The stepwise oxidation of a hydrocarbon by oxygen, in organic chemistry, produces water and, successively: an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
, an aldehyde or a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
, a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, and then a peroxide.
Corrosion and rusting
 * The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of
* The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of whic ...
s, is a well-known example of electrochemical corrosion; it forms as a result of the oxidation of iron metal. Common rust often refers to iron(III) oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally ...
, formed in the following chemical reaction:
::
* The oxidation of iron(II) to iron(III) by hydrogen peroxide in the presence of an acid:
::
::
:Here the overall equation involves adding the reduction equation to twice the oxidation equation, so that the electrons cancel:
::
Disproportionation
A disproportionation reaction is one in which a single substance is both oxidized and reduced. For example, thiosulfate ion with sulfur in oxidation state +2 can react in the presence of acid to form elemental sulfur (oxidation state 0) andsulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
(oxidation state +4).
:
Thus one sulfur atom is reduced from +2 to 0, while the other is oxidized from +2 to +4.
Redox reactions in industry
Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects protected metal to a more easily corroded "sacrificial anode
A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged metal structures from corrosion.
They are made from a metal alloy with a more "active" voltage (more n ...
" to act as the anode. The sacrificial metal instead of the protected metal, then, corrodes. A common application of cathodic protection is in galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.
Oxidation is used in a wide variety of industries such as in the production of cleaning products and oxidizing ammonia to produce nitric acid.
Redox reactions are the foundation of electrochemical cells, which can generate electrical energy or support electrosynthesis. Metal ores often contain metals in oxidized states such as oxides or sulfides, from which the pure metals are extracted by smelting at high temperature in the presence of a reducing agent. The process of electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
uses redox reactions to coat objects with a thin layer of a material, as in chrome-plated
Chrome plating (less commonly chromium plating) is a technique of electroplating a thin layer of chromium onto a metal object. A chrome-plated item is called ''chrome''. The chromed layer can be decorative, provide corrosion resistance, ease o ...
automotive parts, silver plating cutlery
Cutlery (also referred to as silverware, flatware, or tableware), includes any hand implement used in preparing, serving, and especially eating food in Western culture. A person who makes or sells cutlery is called a cutler. The city of Sheffie ...
, galvanization and gold-plated
Gold plating is a method of depositing a thin layer of gold onto the surface of another metal, most often copper or silver (to make silver-gilt), by chemical or electrochemical plating. This article covers plating methods used in the modern ele ...
jewelry.
Redox reactions in biology
Top:
Bottom: dehydroascorbic acid ( oxidized form of Vitamin C)
ascorbic acid
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) an ...
( reduced form of Vitamin C)Bottom: dehydroascorbic acid ( oxidized form of Vitamin C)
 Many important
Many important biological
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary in ...
processes involve redox reactions. Before some of these processes can begin iron must be assimilated from the environment.
Cellular respiration, for instance, is the oxidation of glucose (C6H12O6) to CO2 and the reduction of oxygen to water. The summary equation for cell respiration is:
:
The process of cell respiration also depends heavily on the reduction of NAD+ to NADH and the reverse reaction (the oxidation of NADH to NAD+). Photosynthesis and cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
:
Biological energy is frequently stored and released by means of redox reactions. Photosynthesis involves the reduction of carbon dioxide into sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
s and the oxidation of water into molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reduce nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an aden ...
(NAD+) to NADH, which then contributes to the creation of a proton gradient, which drives the synthesis of adenosine triphosphate (ATP) and is maintained by the reduction of oxygen.
In animal cells, mitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosi ...
perform similar functions. See the ''Membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges ...
'' article.
Free radical reactions are redox reactions that occur as a part of homeostasis and killing microorganisms, where an electron detaches from a molecule and then reattaches almost instantaneously. Free radicals are a part of redox molecules and can become harmful to the human body if they do not reattach to the redox molecule or an antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricant ...
. Unsatisfied free radicals can spur the mutation of cells they encounter and are, thus, causes of cancer.
The term ''redox state'' is often used to describe the balance of GSH/GSSG, NAD+/NADH and NADP+/NADPH in a biological system such as a cell or organ. The redox state is reflected in the balance of several sets of metabolites (e.g., lactate
Lactate may refer to:
* Lactation, the secretion of milk from the mammary glands
* Lactate, the conjugate base of lactic acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with ...
and pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
, beta-hydroxybutyrate
β-Hydroxybutyric acid, also known as 3-hydroxybutyric acid or BHB, is an organic compound and a beta hydroxy acid with the chemical formula CH3CH(OH)CH2CO2H; its conjugate base is β-hydroxybutyrate, also known as 3-hydroxybutyrate. β-Hydroxyb ...
, and acetoacetate
Acetoacetic acid (also acetoacetate and diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stab ...
), whose interconversion is dependent on these ratios. An abnormal redox state can develop in a variety of deleterious situations, such as hypoxia
Hypoxia means a lower than normal level of oxygen, and may refer to:
Reduced or insufficient oxygen
* Hypoxia (environmental), abnormally low oxygen content of the specific environment
* Hypoxia (medical), abnormally low level of oxygen in the tis ...
, shock, and sepsis. Redox mechanism also control some cellular processes. Redox proteins and their genes must be co-located for redox regulation according to the CoRR hypothesis for the function of DNA in mitochondria and chloroplasts.
Redox cycling
Wide varieties of aromatic compounds areenzymatically
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
reduced to form free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
that contain one more electron than their parent compounds. In general, the electron donor is any of a wide variety of flavoenzymes and their coenzymes. Once formed, these anion free radicals reduce molecular oxygen to superoxide and regenerate the unchanged parent compound. The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide. This catalytic behavior has been described as a futile cycle or redox cycling.
Redox reactions in geology
 Minerals are generally oxidized derivatives of metals. Iron is mined as its magnetite (Fe3O4). Titanium is mined as its dioxide, usually in the form of rutile (TiO2). To obtain the corresponding metals, these oxides must be reduced, which is often achieved by heating these oxides with carbon or carbon monoxide as reducing agents.
Minerals are generally oxidized derivatives of metals. Iron is mined as its magnetite (Fe3O4). Titanium is mined as its dioxide, usually in the form of rutile (TiO2). To obtain the corresponding metals, these oxides must be reduced, which is often achieved by heating these oxides with carbon or carbon monoxide as reducing agents. Blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric ...
s are the reactors where iron oxides and coke (a form of carbon) are combined to produce molten iron.The main chemical reaction producing the molten iron is:
:
Redox reactions in soils
Electron transfer reactions are central to myriad processes and properties in soils, and electron "activity", quantified as Eh (platinum electrode potential (voltage) relative to the standard hydrogen electrode) or pe (analogous to pH as -log electron activity), is a master variable, along with pH, that controls and is governed by chemical reactions and biological processes. Early theoretical research with applications to flooded soils and paddy rice production was seminal for subsequent work on thermodynamic aspects of redox and plant root growth in soils. Later work built on this foundation, and expanded it for understanding redox reactions related to heavy metal oxidation state changes, pedogenesis and morphology, organic compound degradation and formation, free radical chemistry, wetland delineation, soil remediation, and various methodological approaches for characterizing the redox status of soils.Mnemonics
The key terms involved in redox can be confusing. For example, a reagent that is oxidized loses electrons; however, that reagent is referred to as the reducing agent. Likewise, a reagent that is reduced gains electrons and is referred to as the oxidizing agent. These mnemonics are commonly used by students to help memorise the terminology: * " OIL RIG" — oxidation is loss of electrons, reduction is gain of electrons * "LEO the lion says GER rr — loss of electrons is oxidation, gain of electrons is reduction * "LEORA says GEROA" — the loss of electrons is called oxidation (reducing agent); the gain of electrons is called reduction (oxidizing agent). * "RED CAT" and "AN OX", or "AnOx RedCat" ("an ox-red cat") — reduction occurs at the cathode and the anode is for oxidation * "RED CAT gains what AN OX loses" – reduction at the cathode gains (electrons) what anode oxidation loses (electrons) * "PANIC" – Positive Anode and Negative is Cathode. This applies to electrolytic cells which release stored electricity, and can be recharged with electricity. PANIC does not apply to cells that can be recharged with redox materials. These galvanic or voltaic cells, such asfuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
s, produce electricity from internal redox reactions. Here, the positive electrode is the cathode and the negative is the anode.
See also
* Anaerobic respiration * Bessemer process * Bioremediation *Calvin cycle
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into ...
* Chemical equation
* Chemical looping combustion
* Citric acid cycle
* Electrochemical series
* Electrochemistry
* Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
* Electron equivalent
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
* Electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
* Electrosynthesis
* Galvanic cell
* Hydrogenation
* Membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges ...
* Microbial fuel cell
* Murburn concept
In the field of enzymology, murburn is a term coined by Kelath Murali Manoj that explains the catalytic mechanism of certain redox-active proteins. The term describes the equilibrium among molecules, unbound ions and radicals, signifying a process ...
* Nucleophilic abstraction
* Organic redox reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carr ...
* Oxidative addition and reductive elimination
* Oxidative phosphorylation
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine tri ...
* Partial oxidation
* Pro-oxidant Pro-oxidants are chemicals that induce oxidative stress, either by generating reactive oxygen species or by inhibiting antioxidant systems. The oxidative stress produced by these chemicals can damage cells and tissues, for example an overdose of th ...
* Redox gradient
* Redox potential
* Reducing agent
* Reducing atmosphere
* Reduction potential
* Thermic reaction
* Transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
* Sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element ( CHNOPS), being a const ...
References
Further reading
* *External links
Chemical Equation Balancer
– An open-source chemical equation balancer that handles redox reactions.
Online redox reaction equation balancer, balances equations of any half-cell and full reactions
{{Authority control Soil chemistry Chemical reactions Articles containing video clips Redox Reaction mechanisms