Glycine Remota on:
[Wikipedia]
[Google]
[Amazon]
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the
 Glycine functions as a
Glycine functions as a
 Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate are used as supplements for animal feeds.
The U.S. "Food and Drug Administration no longer regards glycine and its salts as generally recognized as safe for use in human food".
Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate are used as supplements for animal feeds.
The U.S. "Food and Drug Administration no longer regards glycine and its salts as generally recognized as safe for use in human food".
Glycine MS Spectrum
* *
{{Authority control Flavor enhancers Glucogenic amino acids Inhibitory amino acids Proteinogenic amino acids Glycine receptor agonists NMDA receptor agonists E-number additives Pages including recorded pronunciations
codon
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
s starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen
Collagen () is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole ...
triple-helices. Glycine is also an inhibitory neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
– interference with its release within the spinal cord (such as during a '' Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction.
It is the only achiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.
History and etymology
Glycine was discovered in 1820 by the French chemist Henri Braconnot when he hydrolyzedgelatin
Gelatin or gelatine (from la, gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also ...
by boiling it with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
. He originally called it "sugar of gelatin", but the French chemist Jean-Baptiste Boussingault
Jean-Baptiste Joseph Dieudonné Boussingault (2 February 1801 – 11 May 1887) was a French chemist who made significant contributions to agricultural science, petroleum science and metallurgy.
Biography
Jean-Baptiste Boussingault – an agric ...
showed that it contained nitrogen. The American scientist Eben Norton Horsford, then a student of the German chemist Justus von Liebig
Justus Freiherr von Liebig (12 May 1803 – 20 April 1873) was a German scientist who made major contributions to agricultural and biological chemistry, and is considered one of the principal founders of organic chemistry. As a professor at t ...
, proposed the name "glycocoll"; however, the Swedish
Swedish or ' may refer to:
Anything from or related to Sweden, a country in Northern Europe. Or, specifically:
* Swedish language, a North Germanic language spoken primarily in Sweden and Finland
** Swedish alphabet, the official alphabet used by ...
chemist Berzelius suggested the simpler name "glycine". The name comes from the Greek word γλυκύς "sweet tasting" (which is also related to the prefixes '' glyco-'' and '' gluco-'', as in ''glycoprotein
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycos ...
'' and '' glucose''). In 1858, the French chemist Auguste Cahours determined that glycine was an amine of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
.
Production
Although glycine can be isolated from hydrolyzed protein, this route is not used for industrial production, as it can be manufactured more conveniently by chemical synthesis. The two main processes are amination of chloroacetic acid with ammonia, giving glycine and ammonium chloride, and theStrecker amino acid synthesis
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonia in the presence of potassium cyanide. The condensation reaction yields an α- ...
, which is the main synthetic method in the United States and Japan. About 15 thousand tonnes are produced annually in this way.
Glycine is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct.
Chemical reactions
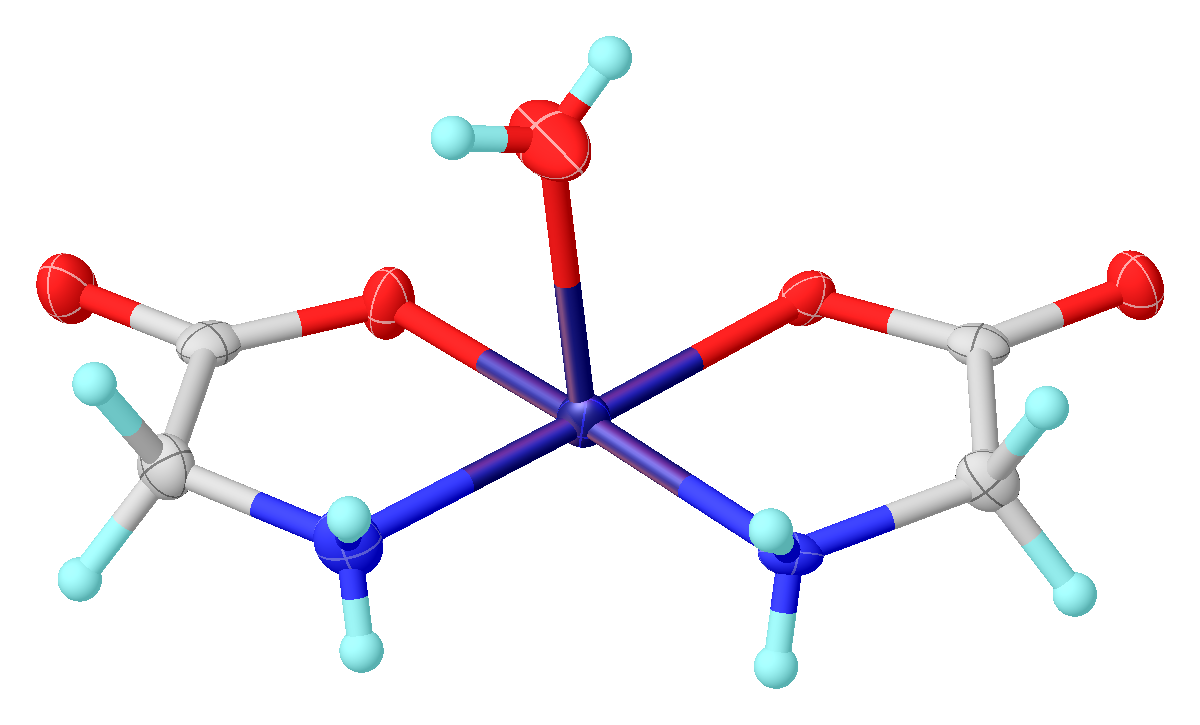
Its acid–base properties are most important. In aqueous solution, glycine is amphoteric: below pH = 2.4, it converts to the ammonium cation called glycinium. Above about 9.6, it converts to glycinate. : Glycine functions as a
Glycine functions as a bidentate ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
for many metal ions, forming amino acid complexes. A typical complex is Cu(glycinate)2, i.e. Cu(H2NCH2CO2)2, which exists both in cis and trans isomers.
With acid chlorides, glycine converts to the amidocarboxylic acid, such as hippuric acid
Hippuric acid ( Gr. ''hippos'', horse, ''ouron'', urine) is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acid and glycine. Levels of hippuric acid rise with the consumption of phenoli ...
and acetylglycine
Aceturic acid (''N''-acetylglycine) is a derivative of the amino acid glycine. The conjugate base of this carboxylic acid is called ''aceturate'', a term used for its esters and salts.
Preparation
Aceturic acid can be prepared by warming glycine ...
. With nitrous acid, one obtains glycolic acid (van Slyke determination The Van Slyke determination is a chemical test for the determination of amino acids containing a primary amine group. It is named after the biochemist Donald Dexter Van Slyke (1883-1971).
One of Van Slyke's first professional achievements was the ...
). With methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
, the amine becomes quaternized to give trimethylglycine, a natural product:
: + 3 CH3I → + 3 HI
Glycine condenses with itself to give peptides, beginning with the formation of glycylglycine:
:2 → + H2O
Pyrolysis of glycine or glycylglycine gives 2,5-diketopiperazine, the cyclic diamide.
It forms esters with alcohols. They are often isolated as their hydrochloride, e.g., glycine methyl ester hydrochloride. Otherwise the free ester tends to convert to diketopiperazine
A diketopiperazine (DKP), also known as a ''dioxopiperazine'' or ''piperazinedione'', is a class of organic compounds related to piperazine but containing two amide linkages. DKP's are the smallest known class of cyclic peptide. Despite their name, ...
.
As a bifunctional molecule, glycine reacts with many reagents. These can be classified into N-centered and carboxylate-center reactions.
Metabolism
Biosynthesis
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acidserine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
, which is in turn derived from 3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. Th ...
, but the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:
: serine + tetrahydrofolate → glycine + ''N5'',''N10''-methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase
The glycine cleavage system (GCS) is also known as the glycine decarboxylase complex or GDC. The system is a series of enzymes that are triggered in response to high concentrations of the amino acid glycine. The same set of enzymes is sometimes r ...
(also called glycine cleavage enzyme). This conversion is readily reversible:
: CO2 + NH + ''N5'',''N10''-methylene tetrahydrofolate + NADH + H+ ⇌ Glycine + tetrahydrofolate + NAD+
In addition to being synthesized from serine, glycine can also be derived from threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO� ...
, choline or hydroxyproline via inter-organ metabolism of the liver and kidneys.
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants is the reverse of the glycine synthase pathway mentioned above. In this context, the enzyme system involved is usually called theglycine cleavage system
The glycine cleavage system (GCS) is also known as the glycine decarboxylase complex or GDC. The system is a series of enzymes that are triggered in response to high concentrations of the amino acid glycine. The same set of enzymes is sometimes r ...
:
: Glycine + tetrahydrofolate + NAD+ ⇌ CO2 + NH + ''N5'',''N10''-methylene tetrahydrofolate + NADH + H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
by serine dehydratase
Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the ...
.
In the third pathway of its degradation, glycine is converted to glyoxylate
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Str ...
by D-amino acid oxidase
D-amino acid oxidase (DAAO; also OXDA, DAMOX) is an enzyme with the function on a molecular level to oxidize D-amino acids to the corresponding α-keto acids, producing ammonia and hydrogen peroxide. This results in a number of physiological e ...
. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction.
The half-life of glycine and its elimination from the body varies significantly based on dose. In one study, the half-life varied between 0.5 and 4.0 hours.
Glycine is extremely sensitive to antibiotics which target folate, and blood glycine levels drop severely within a minute of antibiotic injections. Some antibiotics can deplete more than 90% of glycine within a few minutes of being administered.
Physiological function
The principal function of glycine is it acts as a precursor to proteins. Most proteins incorporate only small quantities of glycine, a notable exception beingcollagen
Collagen () is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole ...
, which contains about 35% glycine due to its periodically repeated role in the formation of collagen's helix structure in conjunction with hydroxyproline. In the genetic code, glycine is coded by all codons
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
starting with GG, namely GGU, GGC, GGA and GGG.
As a biosynthetic intermediate
In highereukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase
Aminolevulinic acid synthase (ALA synthase, ALAS, or delta-aminolevulinic acid synthase) is an enzyme () that catalyzes the synthesis of δ-aminolevulinic acid (ALA) the first common precursor in the biosynthesis of all tetrapyrroles such as hemes ...
. Glycine provides the central C2N subunit of all purines.
As a neurotransmitter
Glycine is an inhibitoryneurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
in the central nervous system, especially in the spinal cord, brainstem
The brainstem (or brain stem) is the posterior stalk-like part of the brain that connects the cerebrum with the spinal cord. In the human brain the brainstem is composed of the midbrain, the pons, and the medulla oblongata. The midbrain is cont ...
, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an inhibitory postsynaptic potential (IPSP). Strychnine
Strychnine (, , US chiefly ) is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eye ...
is a strong antagonist at ionotropic glycine receptors, whereas bicuculline
Bicuculline is a phthalide-isoquinoline compound that is a light-sensitive competitive antagonist of GABAA receptors. It was originally identified in 1932 in plant alkaloid extracts and has been isolated from ''Dicentra cucullaria'', '' Adlumia ...
is a weak one. Glycine is a required co-agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agoni ...
along with glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
for NMDA receptor
The ''N''-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA rece ...
s. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the ( NMDA) glutamatergic receptors which are excitatory. The of glycine is 7930 mg/kg in rats (oral), and it usually causes death by hyperexcitability.
Uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia (“USP”), and technical grade. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine. If purity greater than the USP standard is needed, for example forintravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrie ...
injections, a more expensive pharmaceutical grade glycine can be used. Technical grade glycine, which may or may not meet USP grade standards, is sold at a lower price for use in industrial applications, e.g., as an agent in metal complexing and finishing.
Animal and human foods
 Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate are used as supplements for animal feeds.
The U.S. "Food and Drug Administration no longer regards glycine and its salts as generally recognized as safe for use in human food".
Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate are used as supplements for animal feeds.
The U.S. "Food and Drug Administration no longer regards glycine and its salts as generally recognized as safe for use in human food".
Chemical feedstock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of theherbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page fo ...
s glyphosate, iprodione, glyphosine, imiprothrin
Imiprothrin is a synthetic pyrethroid insecticide. It is an ingredient in some commercial and consumer insecticide products for indoor use. It has low acute toxicity to humans through the inhalation and dermal routes, but to insects it acts as a ...
, and eglinazine. It is used as an intermediate of the medicine such as thiamphenicol
Thiamphenicol (also known as thiophenicol and dextrosulphenidol) is an antibiotic. It is the methyl-sulfonyl analogue of chloramphenicol and has a similar spectrum of activity, but is 2.5 to 5 times as potent. Like chloramphenicol, it is insolubl ...
.
Laboratory research
Glycine is a significant component of some solutions used in the SDS-PAGE method of protein analysis. It serves as a buffering agent, maintaining pH and preventing sample damage during electrophoresis. Glycine is also used to remove protein-labeling antibodies from Western blot membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel. This allows more data to be drawn from the same specimen, increasing the reliability of the data, reducing the amount of sample processing, and number of samples required. This process is known as stripping.Presence in space
The presence of glycine outside the earth was confirmed in 2009, based on the analysis of samples that had been taken in 2004 by the NASA spacecraft ''Stardust
Stardust may refer to:
* A type of cosmic dust, composed of particles in space
Entertainment Songs
* “Stardust” (1927 song), by Hoagy Carmichael
* “Stardust” (David Essex song), 1974
* “Stardust” (Lena Meyer-Landrut song), 2012
* ...
'' from comet Wild 2
Comet 81P/Wild, also known as Wild 2 (pronounced "vilt two") ( ), is a comet named after Swiss astronomer Paul Wild, who discovered it on January 6, 1978, using a 40-cm Schmidt telescope at Zimmerwald, Switzerland.
For most of its 4.5 billion- ...
and subsequently returned to earth. Glycine had previously been identified in the Murchison meteorite in 1970. The discovery of glycine in outer space bolstered the hypothesis of so called soft-panspermia, which claims that the "building blocks" of life are widespread throughout the universe. In 2016, detection of glycine within Comet 67P/Churyumov–Gerasimenko by the ''Rosetta'' spacecraft was announced.
The detection of glycine outside the Solar System in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
has been debated. In 2008, the Max Planck Institute for Radio Astronomy discovered the spectral lines of a glycine precursor ( aminoacetonitrile) in the Large Molecule Heimat, a giant gas cloud near the galactic center in the constellation Sagittarius.
Evolution
Glycine is proposed to be defined by early genetic codes. For example, low complexity regions (in proteins), that may resemble the proto-peptides of the early genetic code are highly enriched in glycine.Presence in foods
See also
* Trimethylglycine *Amino acid neurotransmitter
An amino acid neurotransmitter is an amino acid which is able to transmit a nerve message across a synapse. Neurotransmitters (chemicals) are packaged into vesicles that cluster beneath the axon terminal membrane on the presynaptic side of a syn ...
References
Further reading
* *External links
Glycine MS Spectrum
* *
{{Authority control Flavor enhancers Glucogenic amino acids Inhibitory amino acids Proteinogenic amino acids Glycine receptor agonists NMDA receptor agonists E-number additives Pages including recorded pronunciations