Gas Masks Of The United States on:
[Wikipedia]
[Google]
[Amazon]
 Gas is one of the four fundamental
Gas is one of the four fundamental
 When observing a gas, it is typical to specify a frame of reference or
When observing a gas, it is typical to specify a frame of reference or
 If one could observe a gas under a powerful microscope, one would see a collection of particles without any definite shape or volume that are in more or less random motion. These gas particles only change direction when they collide with another particle or with the sides of the container. This
If one could observe a gas under a powerful microscope, one would see a collection of particles without any definite shape or volume that are in more or less random motion. These gas particles only change direction when they collide with another particle or with the sides of the container. This

 The intermolecular attractions and repulsions between two gas molecules are dependent on the amount of distance between them. The combined attractions and repulsions are well-modelled by the Lennard-Jones potential, which is one of the most extensively studied of all interatomic potentials describing the potential energy of molecular systems. The Lennard-Jones potential between molecules can be broken down into two separate components: a long-distance attraction due to the
The intermolecular attractions and repulsions between two gas molecules are dependent on the amount of distance between them. The combined attractions and repulsions are well-modelled by the Lennard-Jones potential, which is one of the most extensively studied of all interatomic potentials describing the potential energy of molecular systems. The Lennard-Jones potential between molecules can be broken down into two separate components: a long-distance attraction due to the
 Each one of the assumptions listed below adds to the complexity of the problem's solution. As the density of a gas increases with rising pressure, the intermolecular forces play a more substantial role in gas behavior which results in the ideal gas law no longer providing "reasonable" results. At the upper end of the engine temperature ranges (e.g. combustor sections – 1300 K), the complex fuel particles absorb internal energy by means of rotations and vibrations that cause their specific heats to vary from those of diatomic molecules and noble gases. At more than double that temperature, electronic excitation and dissociation of the gas particles begins to occur causing the pressure to adjust to a greater number of particles (transition from gas to plasma). Finally, all of the thermodynamic processes were presumed to describe uniform gases whose velocities varied according to a fixed distribution. Using a non-equilibrium situation implies the flow field must be characterized in some manner to enable a solution. One of the first attempts to expand the boundaries of the ideal gas law was to include coverage for different thermodynamic processes by adjusting the equation to read ''pVn = constant'' and then varying the ''n'' through different values such as the specific heat ratio, ''γ''.
Real gas effects include those adjustments made to account for a greater range of gas behavior:
* Compressibility effects (''Z'' allowed to vary from 1.0)
*Variable
Each one of the assumptions listed below adds to the complexity of the problem's solution. As the density of a gas increases with rising pressure, the intermolecular forces play a more substantial role in gas behavior which results in the ideal gas law no longer providing "reasonable" results. At the upper end of the engine temperature ranges (e.g. combustor sections – 1300 K), the complex fuel particles absorb internal energy by means of rotations and vibrations that cause their specific heats to vary from those of diatomic molecules and noble gases. At more than double that temperature, electronic excitation and dissociation of the gas particles begins to occur causing the pressure to adjust to a greater number of particles (transition from gas to plasma). Finally, all of the thermodynamic processes were presumed to describe uniform gases whose velocities varied according to a fixed distribution. Using a non-equilibrium situation implies the flow field must be characterized in some manner to enable a solution. One of the first attempts to expand the boundaries of the ideal gas law was to include coverage for different thermodynamic processes by adjusting the equation to read ''pVn = constant'' and then varying the ''n'' through different values such as the specific heat ratio, ''γ''.
Real gas effects include those adjustments made to account for a greater range of gas behavior:
* Compressibility effects (''Z'' allowed to vary from 1.0)
*Variable
 Boyle's law was perhaps the first expression of an equation of state. In 1662
Boyle's law was perhaps the first expression of an equation of state. In 1662
 In 1801, John Dalton published the law of partial pressures from his work with ideal gas law relationship: The pressure of a mixture of non reactive gases is equal to the sum of the pressures of all of the constituent gases alone. Mathematically, this can be represented for ''n'' species as:
: Pressuretotal = Pressure1 + Pressure2 + ... + Pressure''n''
The image of Dalton's journal depicts symbology he used as shorthand to record the path he followed. Among his key journal observations upon mixing unreactive "elastic fluids" (gases) were the following:
*Unlike liquids, heavier gases did not drift to the bottom upon mixing.
*Gas particle identity played no role in determining final pressure (they behaved as if their size was negligible).
In 1801, John Dalton published the law of partial pressures from his work with ideal gas law relationship: The pressure of a mixture of non reactive gases is equal to the sum of the pressures of all of the constituent gases alone. Mathematically, this can be represented for ''n'' species as:
: Pressuretotal = Pressure1 + Pressure2 + ... + Pressure''n''
The image of Dalton's journal depicts symbology he used as shorthand to record the path he followed. Among his key journal observations upon mixing unreactive "elastic fluids" (gases) were the following:
*Unlike liquids, heavier gases did not drift to the bottom upon mixing.
*Gas particle identity played no role in determining final pressure (they behaved as if their size was negligible).
 Thermodynamicists use this factor (''Z'') to alter the ideal gas equation to account for compressibility effects of real gases. This factor represents the ratio of actual to ideal specific volumes. It is sometimes referred to as a "fudge-factor" or correction to expand the useful range of the ideal gas law for design purposes. ''Usually'' this ''Z'' value is very close to unity. The compressibility factor image illustrates how Z varies over a range of very cold temperatures.
Thermodynamicists use this factor (''Z'') to alter the ideal gas equation to account for compressibility effects of real gases. This factor represents the ratio of actual to ideal specific volumes. It is sometimes referred to as a "fudge-factor" or correction to expand the useful range of the ideal gas law for design purposes. ''Usually'' this ''Z'' value is very close to unity. The compressibility factor image illustrates how Z varies over a range of very cold temperatures.
 Viscosity, a physical property, is a measure of how well adjacent molecules stick to one another. A solid can withstand a shearing force due to the strength of these sticky intermolecular forces. A fluid will continuously deform when subjected to a similar load. While a gas has a lower value of viscosity than a liquid, it is still an observable property. If gases had no viscosity, then they would not stick to the surface of a wing and form a boundary layer. A study of the delta wing in the
Viscosity, a physical property, is a measure of how well adjacent molecules stick to one another. A solid can withstand a shearing force due to the strength of these sticky intermolecular forces. A fluid will continuously deform when subjected to a similar load. While a gas has a lower value of viscosity than a liquid, it is still an observable property. If gases had no viscosity, then they would not stick to the surface of a wing and form a boundary layer. A study of the delta wing in the
 In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic, stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time. The satellite view of weather around Robinson Crusoe Islands illustrates one example.
In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic, stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time. The satellite view of weather around Robinson Crusoe Islands illustrates one example.
Animated Gas Lab
Accessed February 2008. *Georgia State University
Accessed February 2008. *Antony Lewi
WordWeb
Accessed February 2008. *Northwestern Michigan Colleg
Accessed February 2008. * {{Authority control Gases *Gas Articles containing video clips
 Gas is one of the four fundamental
Gas is one of the four fundamental states of matter
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, ...
(the others being solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural ...
, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
like neon), elemental
An elemental is a mythic being that is described in occult and alchemical works from around the time of the European Renaissance, and particularly elaborated in the 16th century works of Paracelsus. According to Paracelsus and his subsequent fo ...
molecules made from one type of atom (e.g. oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
), or compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
molecules made from a variety of atoms (e.g. carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
). A gas mixture, such as air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
, contains a variety of pure gases. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles
In the physical sciences, a particle (or corpuscule in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
. This separation usually makes a colourless gas invisible to the human observer.
The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention.
High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gas
An ideal Bose gas is a quantum-mechanical phase of matter, analogous to a classical ideal gas. It is composed of bosons, which have an integer value of spin, and abide by Bose–Einstein statistics. The statistical mechanics of bosons were deve ...
es or Fermi gas
An ideal Fermi gas is a state of matter which is an ensemble of many non-interacting fermions. Fermions are particles that obey Fermi–Dirac statistics, like electrons, protons, and neutrons, and, in general, particles with half-integer sp ...
es. For a comprehensive listing of these exotic states of matter see list of states of matter
States of matter are distinguished by changes in the properties of matter associated with external factors like pressure and temperature. States are usually distinguished by a discontinuity in one of those properties—for example, raising the tem ...
.
Elemental gases
The only chemical elements that are stable diatomichomonuclear
Homonuclear molecules, or homonuclear species, are molecules composed of only one element. Homonuclear molecules may consist of various numbers of atoms. The size of the molecule an element can form depends on the element's properties, and some el ...
molecular gases at STP
STP may refer to:
Places
* São Tomé and Príncipe (ISO 3166-1 alpha-3 code, IOC country code, and FIFA country code STP)
* St Pancras railway station, London St Pancras (Domestic) railway station (National Rail code STP)
* St. Paul Downtown Air ...
are hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
(H2), nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
(N2), oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
(O2), and two halogens
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group i ...
: fluorine (F2) and chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
(Cl2). When grouped together with the monatomic
In physics and chemistry, "monatomic" is a combination of the words "mono" and "atomic", and means "single atom". It is usually applied to gases: a monatomic gas is a gas in which atoms are not bound to each other. Examples at standard conditions ...
noble gases
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
– helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
(He), neon (Ne), argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
(Ar), krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often ...
(Kr), xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
(Xe), and radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through ...
(Rn) – these gases are referred to as "elemental gases".
Etymology
The word ''gas'' was first used by the early 17th-centuryFlemish
Flemish (''Vlaams'') is a Low Franconian dialect cluster of the Dutch language. It is sometimes referred to as Flemish Dutch (), Belgian Dutch ( ), or Southern Dutch (). Flemish is native to Flanders, a historical region in northern Belgium; ...
chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe t ...
Jan Baptist van Helmont
Jan Baptist van Helmont (; ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered to b ...
. He identified carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
, the first known gas other than air. Van Helmont's word appears to have been simply a phonetic transcription of the Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic p ...
word χάος ''Chaos
Chaos or CHAOS may refer to:
Arts, entertainment and media Fictional elements
* Chaos (''Kinnikuman'')
* Chaos (''Sailor Moon'')
* Chaos (''Sesame Park'')
* Chaos (''Warhammer'')
* Chaos, in ''Fabula Nova Crystallis Final Fantasy''
* Cha ...
'' – the ''g'' in Dutch
Dutch commonly refers to:
* Something of, from, or related to the Netherlands
* Dutch people ()
* Dutch language ()
Dutch may also refer to:
Places
* Dutch, West Virginia, a community in the United States
* Pennsylvania Dutch Country
People E ...
being pronounced like ''ch'' in "loch" (voiceless velar fricative, ) – in which case Van Helmont was simply following the established alchemical
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
usage first attested in the works of Paracelsus. According to Paracelsus's terminology, ''chaos'' meant something like "ultra-rarefied water".
An alternative story is that Van Helmont's term was derived from "''gahst'' (or ''geist''), which signifies a ghost or spirit". That story is given no credence by the editors of the ''Oxford English Dictionary
The ''Oxford English Dictionary'' (''OED'') is the first and foundational historical dictionary of the English language, published by Oxford University Press (OUP). It traces the historical development of the English language, providing a co ...
''. In contrast, French-American historian Jacques Barzun
Jacques Martin Barzun (; November 30, 1907 – October 25, 2012) was a French-American historian known for his studies of the history of ideas and cultural history. He wrote about a wide range of subjects, including baseball, mystery novels, and ...
speculated that Van Helmont had borrowed the word from the German ''Gäscht'', meaning the froth resulting from fermentation.
Physical characteristics
Because most gases are difficult to observe directly, they are described through the use of four physical properties ormacroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenomena a ...
characteristics: pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
, volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). Th ...
, number of particles
The particle number (or number of particles) of a thermodynamic system, conventionally indicated with the letter ''N'', is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is ...
(chemists group them by moles Moles can refer to:
* Moles de Xert, a mountain range in the Baix Maestrat comarca, Valencian Community, Spain
* The Moles (Australian band)
*The Moles, alter ego of Scottish band Simon Dupree and the Big Sound
People
*Abraham Moles, French engin ...
) and temperature. These four characteristics were repeatedly observed by scientists such as Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
, Jacques Charles
Jacques Alexandre César Charles (November 12, 1746 – April 7, 1823) was a French inventor, scientist, mathematician, and balloonist.
Charles wrote almost nothing about mathematics, and most of what has been credited to him was due to mistaking ...
, John Dalton, Joseph Gay-Lussac and Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (, also , ; 9 August 17769 July 1856) was an Italian scientist, most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volume ...
for a variety of gases in various settings. Their detailed studies ultimately led to a mathematical relationship among these properties expressed by the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
(see simplified models section below).
Gas particles are widely separated from one another, and consequently, have weaker intermolecular bonds than liquids or solids. These intermolecular forces
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
result from electrostatic interactions between gas particles. Like-charged areas of different gas particles repel, while oppositely charged regions of different gas particles attract one another; gases that contain permanently charged ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
are known as plasmas. Gaseous compounds with polar covalent bonds contain permanent charge imbalances and so experience relatively strong intermolecular forces, although the molecule while the compound's net charge remains neutral. Transient, randomly induced charges exist across non-polar covalent bonds of molecules and electrostatic interactions caused by them are referred to as Van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s. The interaction of these intermolecular forces varies within a substance which determines many of the physical properties unique to each gas. A comparison of ''boiling points'' for compounds formed by ionic and covalent bonds leads us to this conclusion. The drifting smoke particles in the image provides some insight into low-pressure gas behavior.
Compared to the other states of matter, gases have low density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
and viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
. Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
and temperature influence the particles within a certain volume. This variation in particle separation and speed is referred to as ''compressibility''. This particle separation and size influences optical properties of gases as can be found in the following list of refractive indices. Finally, gas particles spread apart or diffuse
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
in order to homogeneously distribute themselves throughout any container.
Macroscopic view of gases
 When observing a gas, it is typical to specify a frame of reference or
When observing a gas, it is typical to specify a frame of reference or length scale
In physics, length scale is a particular length or distance determined with the precision of at most a few orders of magnitude. The concept of length scale is particularly important because physical phenomena of different length scales cannot af ...
. A larger length scale corresponds to a macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenomena a ...
or global point of view of the gas. This region (referred to as a volume) must be sufficient in size to contain a large sampling of gas particles. The resulting statistical analysis of this sample size produces the "average" behavior (i.e. velocity, temperature or pressure) of all the gas particles within the region. In contrast, a smaller length scale corresponds to a microscopic
The microscopic scale () is the scale of objects and events smaller than those that can easily be seen by the naked eye, requiring a lens or microscope to see them clearly. In physics, the microscopic scale is sometimes regarded as the scale be ...
or particle point of view.
Macroscopically, the gas characteristics measured are either in terms of the gas particles themselves (velocity, pressure, or temperature) or their surroundings (volume). For example, Robert Boyle studied pneumatic chemistry
In the history of science, pneumatic chemistry is an area of scientific research of the seventeenth, eighteenth, and early nineteenth centuries. Important goals of this work were the understanding of the physical properties of gases and how the ...
for a small portion of his career. One of his experiments related the macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenomena a ...
properties of pressure and volume of a gas. His experiment used a J-tube manometer
Pressure measurement is the measurement of an applied force by a fluid (liquid or gas) on a surface. Pressure is typically measured in units of force per unit of surface area. Many techniques have been developed for the measurement of pressu ...
which looks like a test tube
A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom.
Test tubes are usually placed in ...
in the shape of the letter J. Boyle trapped an inert gas in the closed end of the test tube with a column of mercury, thereby making the number of particles and the temperature constant. He observed that when the pressure was increased in the gas, by adding more mercury to the column, the trapped gas' volume decreased (this is known as an inverse relationship). Furthermore, when Boyle multiplied the pressure and volume of each observation, the product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
was constant. This relationship held for every gas that Boyle observed leading to the law, (PV=k), named to honor his work in this field.
There are many mathematical tools available for analyzing gas properties. As gases are subjected to extreme conditions, these tools become more complex, from the Euler equations
200px, Leonhard Euler (1707–1783)
In mathematics and physics, many topics are named in honor of Swiss mathematician Leonhard Euler (1707–1783), who made many important discoveries and innovations. Many of these items named after Euler include ...
for inviscid flow to the Navier–Stokes equations
In physics, the Navier–Stokes equations ( ) are partial differential equations which describe the motion of viscous fluid substances, named after French engineer and physicist Claude-Louis Navier and Anglo-Irish physicist and mathematician Geo ...
that fully account for viscous effects. These equations are adapted to the conditions of the gas system in question. Boyle's lab equipment allowed the use of algebra
Algebra () is one of the broad areas of mathematics. Roughly speaking, algebra is the study of mathematical symbols and the rules for manipulating these symbols in formulas; it is a unifying thread of almost all of mathematics.
Elementary ...
to obtain his analytical results. His results were possible because he was studying gases in relatively low pressure situations where they behaved in an "ideal" manner. These ideal relationships apply to safety calculations for a variety of flight conditions on the materials in use. The high technology equipment in use today was designed to help us safely explore the more exotic operating environments where the gases no longer behave in an "ideal" manner. This advanced math, including statistics and multivariable calculus
Multivariable calculus (also known as multivariate calculus) is the extension of calculus in one variable to calculus with functions of several variables: the differentiation and integration of functions involving several variables, rather ...
, makes possible the solution to such complex dynamic situations as space vehicle reentry. An example is the analysis of the space shuttle reentry pictured to ensure the material properties under this loading condition are appropriate. In this flight regime, the gas is no longer behaving ideally.
Pressure
The symbol used to represent pressure in equations is "p" or "P" with SI units ofpascals
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined ...
.
When describing a container of gas, the term pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
(or absolute pressure) refers to the average force per unit area that the gas exerts on the surface of the container. Within this volume, it is sometimes easier to visualize the gas particles moving in straight lines until they collide with the container (see diagram at top of the article). The force imparted by a gas particle into the container during this collision is the change in momentum of the particle. During a collision only the normal Normal(s) or The Normal(s) may refer to:
Film and television
* ''Normal'' (2003 film), starring Jessica Lange and Tom Wilkinson
* ''Normal'' (2007 film), starring Carrie-Anne Moss, Kevin Zegers, Callum Keith Rennie, and Andrew Airlie
* ''Norma ...
component of velocity changes. A particle traveling parallel to the wall does not change its momentum. Therefore, the average force on a surface must be the average change in linear momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass a ...
from all of these gas particle collisions.
Pressure is the sum of all the normal components of force exerted by the particles impacting the walls of the container divided by the surface area of the wall.
Temperature
The symbol used to represent ''temperature'' in equations is ''T'' with SI units ofkelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phy ...
s.
The speed of a gas particle is proportional to its absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic w ...
. The volume of the balloon in the video shrinks when the trapped gas particles slow down with the addition of extremely cold nitrogen. The temperature of any physical system is related to the motions of the particles (molecules and atoms) which make up the assystem. In statistical mechanics, temperature is the measure of the average kinetic energy stored in a molecule (also known as the thermal energy). The methods of storing this energy are dictated by the degrees of freedom of the molecule itself ( energy modes). Thermal (kinetic) energy added to a gas or liquid (an endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
process) produces translational, rotational, and vibrational motion. In contrast, a solid can only increase its internal energy by exciting additional vibrational modes, as the crystal lattice structure prevents both translational and rotational motion. These heated gas molecules have a greater speed range (wider distribution of speeds) with a higher average or ''mean'' speed. The variance of this distribution is due to the speeds of individual particles constantly varying, due to repeated collisions with other particles. The speed range can be described by the Maxwell–Boltzmann distribution
In physics (in particular in statistical mechanics), the Maxwell–Boltzmann distribution, or Maxwell(ian) distribution, is a particular probability distribution named after James Clerk Maxwell and Ludwig Boltzmann.
It was first defined and use ...
. Use of this distribution implies ideal gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is a ...
es near thermodynamic equilibrium
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In the ...
for the system of particles being considered.
Specific volume
The symbol used to represent specific volume in equations is "v" with SI units of cubic meters per kilogram. The symbol used to represent volume in equations is "V" with SI units of cubic meters. When performing athermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
analysis, it is typical to speak of intensive and extensive properties. Properties which depend on the amount of gas (either by mass or volume) are called ''extensive'' properties, while properties that do not depend on the amount of gas are called intensive properties. Specific volume is an example of an intensive property because it is the ratio of volume occupied by a ''unit of mass'' of a gas that is identical throughout a system at equilibrium. 1000 atoms a gas occupy the same space as any other 1000 atoms for any given temperature and pressure. This concept is easier to visualize for solids such as iron which are incompressible
In fluid mechanics or more generally continuum mechanics, incompressible flow ( isochoric flow) refers to a flow in which the material density is constant within a fluid parcel—an infinitesimal volume that moves with the flow velocity. An eq ...
compared to gases. However, volume itself --- not specific --- is an extensive property.
Density
The symbol used to represent density in equations is ρ (rho) with SI units of kilograms per cubic meter. This term is thereciprocal
Reciprocal may refer to:
In mathematics
* Multiplicative inverse, in mathematics, the number 1/''x'', which multiplied by ''x'' gives the product 1, also known as a ''reciprocal''
* Reciprocal polynomial, a polynomial obtained from another pol ...
of specific volume.
Since gas molecules can move freely within a container, their mass is normally characterized by density. Density is the amount of mass per unit volume of a substance, or the inverse of specific volume. For gases, the density can vary over a wide range because the particles are free to move closer together when constrained by pressure or volume. This variation of density is referred to as compressibility
In thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility or, if the temperature is held constant, the isothermal compressibility) is a measure of the instantaneous relative volume change of a f ...
. Like pressure and temperature, density is a state variable
A state variable is one of the set of variables that are used to describe the mathematical "state" of a dynamical system. Intuitively, the state of a system describes enough about the system to determine its future behaviour in the absence of a ...
of a gas and the change in density during any process is governed by the laws of thermodynamics. For a static gas, the density is the same throughout the entire container. Density is therefore a scalar quantity. It can be shown by kinetic theory that the density is inversely proportional to the size of the container in which a fixed mass of gas is confined. In this case of a fixed mass, the density decreases as the volume increases.
Microscopic view of gases
microscopic
The microscopic scale () is the scale of objects and events smaller than those that can easily be seen by the naked eye, requiring a lens or microscope to see them clearly. In physics, the microscopic scale is sometimes regarded as the scale be ...
view of gas is well-described by statistical mechanics, but it can be described by many different theories. The ''kinetic theory of gases'', which makes the assumption that these collisions are perfectly elastic
Elastic is a word often used to describe or identify certain types of elastomer, elastic used in garments or stretchable fabrics.
Elastic may also refer to:
Alternative name
* Rubber band, ring-shaped band of rubber used to hold objects togeth ...
, does not account for intermolecular forces of attraction and repulsion.
Kinetic theory of gases
Kinetic theory provides insight into the macroscopic properties of gases by considering their molecular composition and motion. Starting with the definitions of momentum andkinetic energy
In physics, the kinetic energy of an object is the energy that it possesses due to its motion.
It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acc ...
, one can use the conservation of momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass an ...
and geometric relationships of a cube to relate macroscopic system properties of temperature and pressure to the microscopic property of kinetic energy per molecule. The theory provides averaged values for these two properties.
The ''kinetic theory of gases'' can help explain how the system (the collection of gas particles being considered) responds to changes in temperature, with a corresponding change in ''kinetic energy''.
For example: Imagine you have a sealed container of a fixed-size (a ''constant'' volume), containing a fixed-number of gas particles; starting from absolute zero (the theoretical temperature at which atoms or molecules have no thermal energy, i.e. are not moving or vibrating), you begin to add energy to the system by heating the container, so that energy transfers to the particles inside. Once their internal energy is above zero-point energy
Zero-point energy (ZPE) is the lowest possible energy that a quantum mechanical system may have. Unlike in classical mechanics, quantum systems constantly fluctuate in their lowest energy state as described by the Heisenberg uncertainty pri ...
, meaning their ''kinetic'' energy (also known as ''thermal'' energy) is non-zero, the gas particles will begin to move around the container. As the box is further heated (as more energy is added), the individual particles increase their average speed as the system's total internal energy increases. The higher average-speed of all the particles leads to a greater rate at which collisions happen (i.e. greater number of collisions per unit of time), between particles and the container, as well as between the particles themselves.
The ''macro''scopic, measurable quantity of ''pressure,'' is the direct result of these ''micro''scopic particle collisions with the surface, over which, individual molecules exert a small force, each contributing to the total force applied within a specific area. (''Read "''Pressure''" in the above section "''Macroscopic view of gases''".)''
Likewise, the macroscopically measurable quantity of ''temperature'', is a quantification of the overall amount of ''motion, or kinetic energy'' that the particles exhibit. (''Read "''Temperature''" in the above section "''Macroscopic view of gases''".)''
Thermal motion and statistical mechanics
In the ''kinetic theory of gases'', kinetic energy is assumed to purely consist of linear translations according to a speed distribution of ''particles'' in the system. However, in ''real gases'' and other real substances, the motions which define the kinetic energy of a system (which collectively determine the temperature), are much more complex than simple lineartranslation
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
due to the more complex structure of molecules, compared to single atoms which act similarly to point-masses. In real thermodynamic systems, quantum phenomena play a large role in determining thermal motions. The random, thermal motions (kinetic energy) in molecules is a combination of a finite set of possible motions including translation, rotation, and vibration
Vibration is a mechanical phenomenon whereby oscillations occur about an equilibrium point. The word comes from Latin ''vibrationem'' ("shaking, brandishing"). The oscillations may be periodic, such as the motion of a pendulum—or random, su ...
. This finite range of possible motions, along with the finite set of molecules in the system, leads to a finite number of ''microstates
A microstate or ministate is a sovereign state having a very small population or very small land area, usually both. However, the meanings of "state" and "very small" are not well-defined in international law.Warrington, E. (1994). "Lilliputs ...
'' within the system; we call the set of all microstates an ''ensemble
Ensemble may refer to:
Art
* Architectural ensemble
* Ensemble (album), ''Ensemble'' (album), Kendji Girac 2015 album
* Ensemble (band), a project of Olivier Alary
* Ensemble cast (drama, comedy)
* Ensemble (musical theatre), also known as the ...
.'' Specific to atomic or molecular systems, we could potentially have three different kinds of ensemble, depending on the situation: microcanonical ensemble
In statistical mechanics, the microcanonical ensemble is a statistical ensemble that represents the possible states of a mechanical system whose total energy is exactly specified. The system is assumed to be isolated in the sense that it canno ...
, canonical ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the heat ...
, or grand canonical ensemble
In statistical mechanics, the grand canonical ensemble (also known as the macrocanonical ensemble) is the statistical ensemble that is used to represent the possible states of a mechanical system of particles that are in thermodynamic equilibriu ...
. Specific combinations of microstates within an ensemble are how we truly define ''macrostate'' of the system (temperature, pressure, energy, etc.). In order to do that, we must first count all microstates though use of a '' partition function.'' The use of statistical mechanics and the partition function is an important tool throughout all of physical chemistry, because it is the key to connection between the microscopic states of a system and the macroscopic variables which we can measure, such as temperature, pressure, heat capacity, internal energy, enthalpy, and entropy, just to name a few. (''Read'': Partition function Meaning and significance)
Using the partition function to find the energy of a molecule, or system of molecules, can sometimes be approximated by the Equipartition theorem
In classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. T ...
, which greatly-simplifies calculation. However, this method assumes all molecular degrees of freedom are equally populated, and therefore equally utilized for storing energy within the molecule. It would imply that internal energy changes linearly with temperature, which is not the case. This ignores the fact that heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
changes with temperature, due to certain degrees of freedom being unreachable (a.k.a. "frozen out") at lower temperatures. As internal energy of molecules increases, so does the ability to store energy within additional degrees of freedom. As more degrees of freedom become available to hold energy, this causes the molar heat capacity of the substance to increase.
Brownian motion
Brownian motion is the mathematical model used to describe the random movement of particles suspended in a fluid. The gas particle animation, using pink and green particles, illustrates how this behavior results in the spreading out of gases (entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
). These events are also described by particle theory
Particle physics or high energy physics is the study of fundamental particles and forces that constitute matter and radiation. The fundamental particles in the universe are classified in the Standard Model as fermions (matter particles) and b ...
.
Since it is at the limit of (or beyond) current technology to observe individual gas particles (atoms or molecules), only theoretical calculations give suggestions about how they move, but their motion is different from Brownian motion because Brownian motion involves a smooth drag due to the frictional force of many gas molecules, punctuated by violent collisions of an individual (or several) gas molecule(s) with the particle. The particle (generally consisting of millions or billions of atoms) thus moves in a jagged course, yet not so jagged as would be expected if an individual gas molecule were examined.
Intermolecular forces - the primary difference between ''Real'' and ''Ideal'' gases
Forces between two or more molecules or atoms, either attractive or repulsive, are called ''intermolecular forces''. Intermolecular forces are experienced by molecules when they are within physical proximity of one another. These forces are very important for properly modeling molecular systems, as to accurately predict the microscopic behavior of molecules in ''any'' system, and therefore, are necessary for accurately predicting the physical properties of gases (and liquids) across wide variations in physical conditions. Arising from the study ofphysical chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistica ...
, one of the most prominent intermolecular forces throughout physics, are ''van der Waals forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
''. Van der Waals forces play a key role in determining nearly all physical properties of fluids such as viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
, flow rate, and gas dynamics
Compressible flow (or gas dynamics) is the branch of fluid mechanics that deals with flows having significant changes in fluid density. While all flows are compressible, flows are usually treated as being incompressible when the Mach number (the ...
(see physical characteristics section). The van der Waals interactions between gas molecules, is the reason why modeling a "real gas" is more mathematically difficult than an "''ideal'' gas". Ignoring these proximity-dependent forces allows a real gas
Real gases are nonideal gases whose molecules occupy space and have interactions; consequently, they do not adhere to the ideal gas law.
To understand the behaviour of real gases, the following must be taken into account:
*compressibility effect ...
to be treated like an ideal gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is a ...
, which greatly simplifies calculation.
 The intermolecular attractions and repulsions between two gas molecules are dependent on the amount of distance between them. The combined attractions and repulsions are well-modelled by the Lennard-Jones potential, which is one of the most extensively studied of all interatomic potentials describing the potential energy of molecular systems. The Lennard-Jones potential between molecules can be broken down into two separate components: a long-distance attraction due to the
The intermolecular attractions and repulsions between two gas molecules are dependent on the amount of distance between them. The combined attractions and repulsions are well-modelled by the Lennard-Jones potential, which is one of the most extensively studied of all interatomic potentials describing the potential energy of molecular systems. The Lennard-Jones potential between molecules can be broken down into two separate components: a long-distance attraction due to the London dispersion force
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between a ...
, and a short-range repulsion due to electron-electron exchange interaction
In chemistry and physics, the exchange interaction (with an exchange energy and exchange term) is a quantum mechanical effect that only occurs between identical particles. Despite sometimes being called an exchange force in an analogy to classic ...
(which is related to the Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulat ...
).
When two molecules are relatively distant (meaning they have a high ''potential'' energy), they experience a weak attracting force, causing them to move toward each other, lowering their potential energy. However, if the molecules are ''too far'' away, then they would not experience attractive force of any significance. Additionally, if the molecules get ''too close'' then they will collide, and experience a ''very high'' repulsive force (modelled by Hard spheres
Hard spheres are widely used as model particles in the statistical mechanical theory of fluids and solids. They are defined simply as impenetrable spheres that cannot overlap in space. They mimic the extremely strong ("infinitely elastic bouncing" ...
) which is a ''much stronger force'' than the attractions, so that any attraction due to proximity is disregarded.
As two molecules approach each other, from a distance that is ''neither'' too-far, ''nor'' too-close, their attraction increases as the magnitude of their potential energy increases (becoming more negative), and lowers their total internal energy. The attraction causing the molecules to get closer, can only happen if the molecules remain in proximity for the duration of time it takes to physically ''move'' closer. Therefore, the attractive forces are strongest when the molecules move at ''low speeds''. This means that the attraction between molecules is ''significant'' when gas temperatures is ''low''. However, if you were to isothermally compress this cold gas into a small volume, ''forcing'' the molecules into close proximity, and raising the pressure, the repulsions will begin to dominate over the attractions, as the rate at which collisions are happening will increase significantly. Therefore, at low temperatures, and low pressures, ''attraction'' is the dominant intermolecular interaction.
If two molecules are moving at high speeds, in arbitrary directions, along non-intersecting paths, then they will not spend enough time in proximity to be affected by the attractive London-dispersion force. If the two molecules collide, they are moving too fast and their kinetic energy will be much greater than any attractive potential energy, so they will only experience repulsion upon colliding. Thus, attractions between molecules can be neglected at ''high temperatures'' due to high speeds. At high temperatures, and high pressures, ''repulsion'' is the dominant intermolecular interaction.
Accounting for the above stated effects which cause these attractions and repulsions, real gases, delineate from the ''ideal gas'' model by the following generalization:
* At low temperatures, and low pressures, the volume occupied by a real gas, is ''less than'' the volume predicted by the ideal gas law.
* At high temperatures, and high pressures, the volume occupied by a real gas, is ''greater than'' the volume predicted by the ideal gas law.
Mathematical models
An ''equation of state'' (for gases) is a mathematical model used to roughly describe or predict the state properties of a gas. At present, there is no single equation of state that accurately predicts the properties of all gases under all conditions. Therefore, a number of much more accurate equations of state have been developed for gases in specific temperature and pressure ranges. The "gas models" that are most widely discussed are "perfect gas", "ideal gas" and "real gas". Each of these models has its own set of assumptions to facilitate the analysis of a given thermodynamic system. Each successive model expands the temperature range of coverage to which it applies.Ideal and perfect gas
Theequation of state
In physics, chemistry, and thermodynamics, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or intern ...
for an ideal or perfect gas is the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
and reads
:
where ''P'' is the pressure, ''V'' is the volume, ''n'' is amount of gas (in mol units), ''R'' is the universal gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
, 8.314 J/(mol K), and ''T'' is the temperature. Written this way, it is sometimes called the "chemist's version", since it emphasizes the number of molecules ''n''. It can also be written as
:
where is the specific gas constant for a particular gas, in units J/(kg K), and ρ = m/V is density. This notation is the "gas dynamicist's" version, which is more practical in modeling of gas flows involving acceleration without chemical reactions.
The ideal gas law does not make an assumption about the specific heat of a gas. In the most general case, the specific heat is a function of both temperature and pressure. If the pressure-dependence is neglected (and possibly the temperature-dependence as well) in a particular application, sometimes the gas is said to be a perfect gas
In physics and engineering, a perfect gas is a theoretical gas model that differs from real gases in specific ways that makes certain calculations easier to handle. In all perfect gas models, intermolecular forces are neglected. This means that one ...
, although the exact assumptions may vary depending on the author and/or field of science.
For an ideal gas, the ideal gas law applies without restrictions on the specific heat. An ideal gas is a simplified "real gas" with the assumption that the compressibility factor
In thermodynamics, the compressibility factor (Z), also known as the compression factor or the gas deviation factor, describes the deviation of a real gas from ideal gas behaviour. It is simply defined as the ratio of the molar volume of a gas to ...
''Z'' is set to 1 meaning that this pneumatic ratio remains constant. A compressibility factor of one also requires the four state variables to follow the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
.
This approximation is more suitable for applications in engineering although simpler models can be used to produce a "ball-park" range as to where the real solution should lie. An example where the "ideal gas approximation" would be suitable would be inside a combustion chamber
A combustion chamber is part of an internal combustion engine in which the fuel/air mix is burned. For steam engines, the term has also been used for an extension of the firebox which is used to allow a more complete combustion process.
Intern ...
of a jet engine. It may also be useful to keep the elementary reactions and chemical dissociations for calculating emissions.
Real gas
 Each one of the assumptions listed below adds to the complexity of the problem's solution. As the density of a gas increases with rising pressure, the intermolecular forces play a more substantial role in gas behavior which results in the ideal gas law no longer providing "reasonable" results. At the upper end of the engine temperature ranges (e.g. combustor sections – 1300 K), the complex fuel particles absorb internal energy by means of rotations and vibrations that cause their specific heats to vary from those of diatomic molecules and noble gases. At more than double that temperature, electronic excitation and dissociation of the gas particles begins to occur causing the pressure to adjust to a greater number of particles (transition from gas to plasma). Finally, all of the thermodynamic processes were presumed to describe uniform gases whose velocities varied according to a fixed distribution. Using a non-equilibrium situation implies the flow field must be characterized in some manner to enable a solution. One of the first attempts to expand the boundaries of the ideal gas law was to include coverage for different thermodynamic processes by adjusting the equation to read ''pVn = constant'' and then varying the ''n'' through different values such as the specific heat ratio, ''γ''.
Real gas effects include those adjustments made to account for a greater range of gas behavior:
* Compressibility effects (''Z'' allowed to vary from 1.0)
*Variable
Each one of the assumptions listed below adds to the complexity of the problem's solution. As the density of a gas increases with rising pressure, the intermolecular forces play a more substantial role in gas behavior which results in the ideal gas law no longer providing "reasonable" results. At the upper end of the engine temperature ranges (e.g. combustor sections – 1300 K), the complex fuel particles absorb internal energy by means of rotations and vibrations that cause their specific heats to vary from those of diatomic molecules and noble gases. At more than double that temperature, electronic excitation and dissociation of the gas particles begins to occur causing the pressure to adjust to a greater number of particles (transition from gas to plasma). Finally, all of the thermodynamic processes were presumed to describe uniform gases whose velocities varied according to a fixed distribution. Using a non-equilibrium situation implies the flow field must be characterized in some manner to enable a solution. One of the first attempts to expand the boundaries of the ideal gas law was to include coverage for different thermodynamic processes by adjusting the equation to read ''pVn = constant'' and then varying the ''n'' through different values such as the specific heat ratio, ''γ''.
Real gas effects include those adjustments made to account for a greater range of gas behavior:
* Compressibility effects (''Z'' allowed to vary from 1.0)
*Variable heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
(specific heats vary with temperature)
*Van der Waals forces (related to compressibility, can substitute other equations of state)
* Non-equilibrium thermodynamic effects
*Issues with molecular dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
and elementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be elementary if no reaction ...
s with variable composition.
For most applications, such a detailed analysis is excessive. Examples where real gas effects would have a significant impact would be on the Space Shuttle
The Space Shuttle is a retired, partially reusable low Earth orbital spacecraft system operated from 1981 to 2011 by the U.S. National Aeronautics and Space Administration (NASA) as part of the Space Shuttle program. Its official program ...
re-entry
Atmospheric entry is the movement of an object from outer space into and through the gases of an atmosphere of a planet, dwarf planet, or natural satellite. There are two main types of atmospheric entry: ''uncontrolled entry'', such as the entr ...
where extremely high temperatures and pressures were present or the gases produced during geological events as in the image of the 1990 eruption of Mount Redoubt
Redoubt Volcano, or Mount Redoubt ( Dena'ina: ''Bentuggezh K’enulgheli''), is an active stratovolcano in the largely volcanic Aleutian Range of the U.S. state of Alaska. Located at the head of the Chigmit Mountains subrange in Lake Clark N ...
.
Permanent gas
Permanent gas is a term used for a gas which has a critical temperature below the range of normal human-habitable temperatures and therefore cannot be liquefied by pressure within this range. Historically such gases were thought to be impossible to liquefy and would therefore permanently remain in the gaseous state. The term is relevant to ambient temperature storage and transport of gases at high pressure.Historical research
Boyle's law
 Boyle's law was perhaps the first expression of an equation of state. In 1662
Boyle's law was perhaps the first expression of an equation of state. In 1662 Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
performed a series of experiments employing a J-shaped glass tube, which was sealed on one end. Mercury was added to the tube, trapping a fixed quantity of air in the short, sealed end of the tube. Then the volume of gas was carefully measured as additional mercury was added to the tube. The pressure of the gas could be determined by the difference between the mercury level in the short end of the tube and that in the long, open end. The image of Boyle's equipment shows some of the exotic tools used by Boyle during his study of gases.
Through these experiments, Boyle noted that the pressure exerted by a gas held at a constant temperature varies inversely with the volume of the gas. For example, if the volume is halved, the pressure is doubled; and if the volume is doubled, the pressure is halved. Given the inverse relationship between pressure and volume, the product of pressure (''P'') and volume (''V'') is a constant (''k'') for a given mass of confined gas as long as the temperature is constant. Stated as a formula, thus is:
:
Because the before and after volumes and pressures of the fixed amount of gas, where the before and after temperatures are the same both equal the constant ''k'', they can be related by the equation:
Charles's law
In 1787, the French physicist and balloon pioneer,Jacques Charles
Jacques Alexandre César Charles (November 12, 1746 – April 7, 1823) was a French inventor, scientist, mathematician, and balloonist.
Charles wrote almost nothing about mathematics, and most of what has been credited to him was due to mistaking ...
, found that oxygen, nitrogen, hydrogen, carbon dioxide, and air expand to the same extent over the same 80 kelvin interval. He noted that, for an ideal gas at constant pressure, the volume is directly proportional to its temperature:
:
Gay-Lussac's law
In 1802,Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac (, , ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen (with Alexander von Humboldt), for two laws ...
published results of similar, though more extensive experiments. Gay-Lussac credited Charles' earlier work by naming the law in his honor. Gay-Lussac himself is credited with the law describing pressure, which he found in 1809. It states that the pressure exerted on a container's sides by an ideal gas is proportional to its temperature.
:
Avogadro's law
In 1811, Amedeo Avogadro verified that equal volumes of pure gases contain the same number of particles. His theory was not generally accepted until 1858 when another Italian chemist Stanislao Cannizzaro was able to explain non-ideal exceptions. For his work with gases a century prior, the physical constant that bears his name (theAvogadro constant
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining c ...
) is the number of atoms per mole of elemental carbon-12 (). This specific number of gas particles, at standard temperature and pressure (ideal gas law) occupies 22.40 liters, which is referred to as the molar volume
In chemistry and related fields, the molar volume, symbol ''V''m, or \tilde V of a substance is the ratio of the volume occupied by a substance to the amount of substance, usually given at a given temperature and pressure. It is equal to the molar ...
.
Avogadro's law states that the volume occupied by an ideal gas is proportional to the amount of substance in the volume. This gives rise to the molar volume
In chemistry and related fields, the molar volume, symbol ''V''m, or \tilde V of a substance is the ratio of the volume occupied by a substance to the amount of substance, usually given at a given temperature and pressure. It is equal to the molar ...
of a gas, which at STP
STP may refer to:
Places
* São Tomé and Príncipe (ISO 3166-1 alpha-3 code, IOC country code, and FIFA country code STP)
* St Pancras railway station, London St Pancras (Domestic) railway station (National Rail code STP)
* St. Paul Downtown Air ...
is 22.4 dm3/mol (liters per mole). The relation is given by
where ''n'' is the amount of substance of gas (the number of molecules divided by the Avogadro constant
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining c ...
).
Dalton's law
 In 1801, John Dalton published the law of partial pressures from his work with ideal gas law relationship: The pressure of a mixture of non reactive gases is equal to the sum of the pressures of all of the constituent gases alone. Mathematically, this can be represented for ''n'' species as:
: Pressuretotal = Pressure1 + Pressure2 + ... + Pressure''n''
The image of Dalton's journal depicts symbology he used as shorthand to record the path he followed. Among his key journal observations upon mixing unreactive "elastic fluids" (gases) were the following:
*Unlike liquids, heavier gases did not drift to the bottom upon mixing.
*Gas particle identity played no role in determining final pressure (they behaved as if their size was negligible).
In 1801, John Dalton published the law of partial pressures from his work with ideal gas law relationship: The pressure of a mixture of non reactive gases is equal to the sum of the pressures of all of the constituent gases alone. Mathematically, this can be represented for ''n'' species as:
: Pressuretotal = Pressure1 + Pressure2 + ... + Pressure''n''
The image of Dalton's journal depicts symbology he used as shorthand to record the path he followed. Among his key journal observations upon mixing unreactive "elastic fluids" (gases) were the following:
*Unlike liquids, heavier gases did not drift to the bottom upon mixing.
*Gas particle identity played no role in determining final pressure (they behaved as if their size was negligible).
Special topics
Compressibility
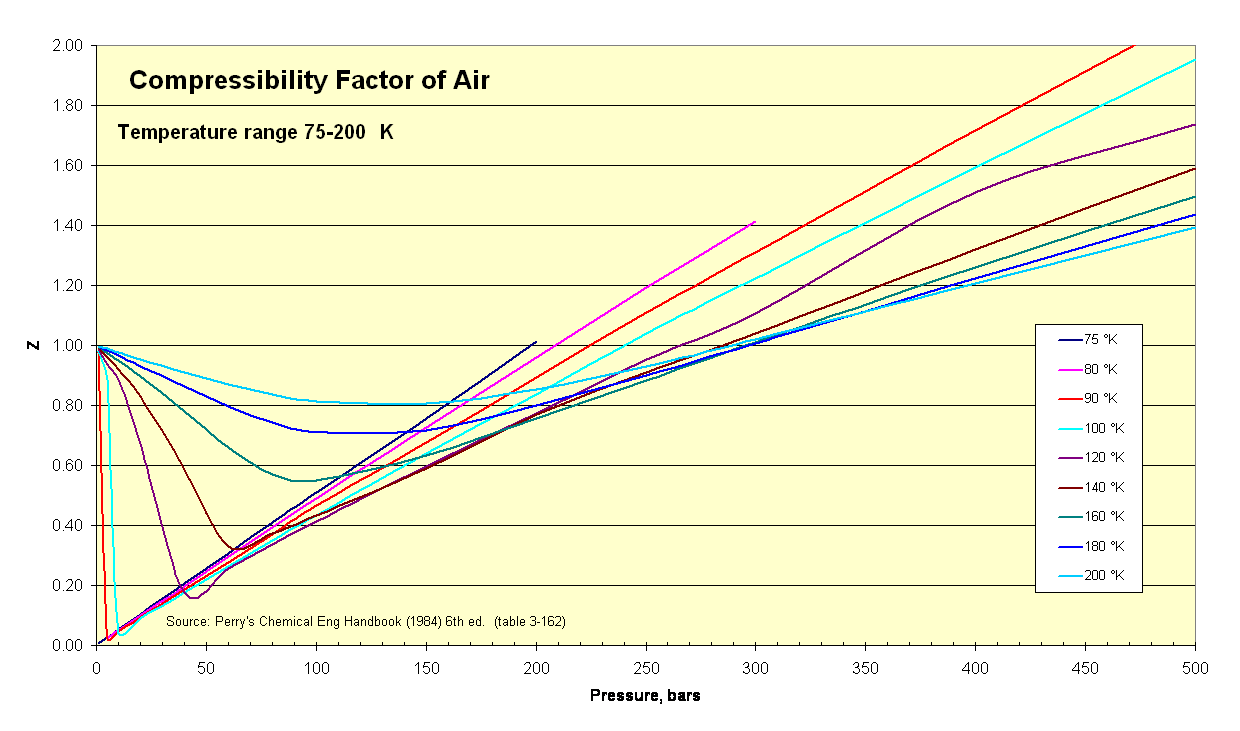
 Thermodynamicists use this factor (''Z'') to alter the ideal gas equation to account for compressibility effects of real gases. This factor represents the ratio of actual to ideal specific volumes. It is sometimes referred to as a "fudge-factor" or correction to expand the useful range of the ideal gas law for design purposes. ''Usually'' this ''Z'' value is very close to unity. The compressibility factor image illustrates how Z varies over a range of very cold temperatures.
Thermodynamicists use this factor (''Z'') to alter the ideal gas equation to account for compressibility effects of real gases. This factor represents the ratio of actual to ideal specific volumes. It is sometimes referred to as a "fudge-factor" or correction to expand the useful range of the ideal gas law for design purposes. ''Usually'' this ''Z'' value is very close to unity. The compressibility factor image illustrates how Z varies over a range of very cold temperatures.
Reynolds number
In fluid mechanics, the Reynolds number is the ratio of inertial forces (''vsρ'') to viscous forces (''μ/L''). It is one of the most important dimensionless numbers in fluid dynamics and is used, usually along with other dimensionless numbers, to provide a criterion for determining dynamic similitude. As such, the Reynolds number provides the link between modeling results (design) and the full-scale actual conditions. It can also be used to characterize the flow.Viscosity
 Viscosity, a physical property, is a measure of how well adjacent molecules stick to one another. A solid can withstand a shearing force due to the strength of these sticky intermolecular forces. A fluid will continuously deform when subjected to a similar load. While a gas has a lower value of viscosity than a liquid, it is still an observable property. If gases had no viscosity, then they would not stick to the surface of a wing and form a boundary layer. A study of the delta wing in the
Viscosity, a physical property, is a measure of how well adjacent molecules stick to one another. A solid can withstand a shearing force due to the strength of these sticky intermolecular forces. A fluid will continuously deform when subjected to a similar load. While a gas has a lower value of viscosity than a liquid, it is still an observable property. If gases had no viscosity, then they would not stick to the surface of a wing and form a boundary layer. A study of the delta wing in the Schlieren
Schlieren ( ; , ) are optical inhomogeneities in transparent media that are not necessarily visible to the human eye. Schlieren physics developed out of the need to produce high-quality lenses devoid of such inhomogeneities. These inhomogeneiti ...
image reveals that the gas particles stick to one another (see Boundary layer section).
Turbulence
 In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic, stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time. The satellite view of weather around Robinson Crusoe Islands illustrates one example.
In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic, stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time. The satellite view of weather around Robinson Crusoe Islands illustrates one example.
Boundary layer
Particles will, in effect, "stick" to the surface of an object moving through it. This layer of particles is called the boundary layer. At the surface of the object, it is essentially static due to the friction of the surface. The object, with its boundary layer is effectively the new shape of the object that the rest of the molecules "see" as the object approaches. This boundary layer can separate from the surface, essentially creating a new surface and completely changing the flow path. The classical example of this is a stalling airfoil. The delta wing image clearly shows the boundary layer thickening as the gas flows from right to left along the leading edge.Maximum entropy principle
As the total number of degrees of freedom approaches infinity, the system will be found in themacrostate
In statistical mechanics, a microstate is a specific microscopic configuration of a thermodynamic system that the system may occupy with a certain probability in the course of its thermal fluctuations. In contrast, the macrostate of a system refe ...
that corresponds to the highest multiplicity
Multiplicity may refer to: In science and the humanities
* Multiplicity (mathematics), the number of times an element is repeated in a multiset
* Multiplicity (philosophy), a philosophical concept
* Multiplicity (psychology), having or using mult ...
. In order to illustrate this principle, observe the skin temperature of a frozen metal bar. Using a thermal image of the skin temperature, note the temperature distribution on the surface. This initial observation of temperature represents a " microstate". At some future time, a second observation of the skin temperature produces a second microstate. By continuing this observation process, it is possible to produce a series of microstates that illustrate the thermal history of the bar's surface. Characterization of this historical series of microstates is possible by choosing the macrostate that successfully classifies them all into a single grouping.
Thermodynamic equilibrium
When energy transfer ceases from a system, this condition is referred to as thermodynamic equilibrium. Usually, this condition implies the system and surroundings are at the same temperature so that heat no longer transfers between them. It also implies that external forces are balanced (volume does not change), and all chemical reactions within the system are complete. The timeline varies for these events depending on the system in question. A container of ice allowed to melt at room temperature takes hours, while in semiconductors the heat transfer that occurs in the device transition from an on to off state could be on the order of a few nanoseconds.See also
* Greenhouse gas * List of gases *Natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
*Volcanic gas
Volcanic gases are gases given off by active (or, at times, by dormant) volcanoes. These include gases trapped in cavities (vesicles) in volcanic rocks, dissolved or dissociated gases in magma and lava, or gases emanating from lava, from volcani ...
*Breathing gas
A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and only natural breathing gas, but other mixtures of gases, or pure oxygen, are also used in breathing equipment and enclosed ...
*Wind
Wind is the natural movement of air or other gases relative to a planet's surface. Winds occur on a range of scales, from thunderstorm flows lasting tens of minutes, to local breezes generated by heating of land surfaces and lasting a few ho ...
Notes
References
* * *Further reading
*Philip Hill and Carl Peterson. ''Mechanics and Thermodynamics of Propulsion: Second Edition'' Addison-Wesley, 1992. *National Aeronautics and Space Administration (NASA)Animated Gas Lab
Accessed February 2008. *Georgia State University
Accessed February 2008. *Antony Lewi
WordWeb
Accessed February 2008. *Northwestern Michigan Colleg
Accessed February 2008. * {{Authority control Gases *Gas Articles containing video clips