Förster Resonance Energy Transfer on:
[Wikipedia]
[Google]
[Amazon]
 Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules ( chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance.
Measurements of FRET efficiency can be used to determine if two
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules ( chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance.
Measurements of FRET efficiency can be used to determine if two

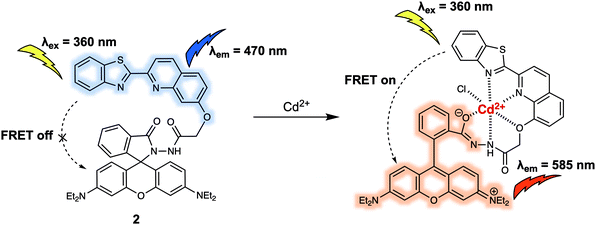 FRET-based probes can detect the presence of various molecules: the probe's structure is affected by small molecule binding or activity, which can turn the FRET system on or off. This is often used to detect anions, cations, small uncharged molecules, and some larger biomacromolecules as well. Similarly, FRET systems have been designed to detect changes in the cellular environment due to such factors as pH, hypoxia, or mitochondrial membrane potential.
FRET-based probes can detect the presence of various molecules: the probe's structure is affected by small molecule binding or activity, which can turn the FRET system on or off. This is often used to detect anions, cations, small uncharged molecules, and some larger biomacromolecules as well. Similarly, FRET systems have been designed to detect changes in the cellular environment due to such factors as pH, hypoxia, or mitochondrial membrane potential.
FRET Imaging
(Tutorial of Becker & Hickl, website) {{DEFAULTSORT:Forster Resonance Energy Transfer Imaging Fluorescence Biochemistry methods Biophysics Cell imaging Optical phenomena Protein–protein interaction assays Fluorescence techniques Cell biology Laboratory techniques Molecular biology techniques Energy transfer
fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
s are within a certain distance of each other. Such measurements are used as a research tool in fields including biology and chemistry.
FRET is analogous to near-field communication, in that the radius of interaction is much smaller than the wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
of light emitted. In the near-field region, the excited chromophore emits a virtual photon
A virtual particle is a theoretical transient particle that exhibits some of the characteristics of an ordinary particle, while having its existence limited by the uncertainty principle. The concept of virtual particles arises in the perturbat ...
that is instantly absorbed by a receiving chromophore. These virtual photons are undetectable, since their existence violates the conservation of energy and momentum, and hence FRET is known as a ''radiationless'' mechanism. Quantum electrodynamical calculations have been used to determine that radiationless (FRET) and radiative energy transfer are the short- and long-range asymptote
In analytic geometry, an asymptote () of a curve is a line such that the distance between the curve and the line approaches zero as one or both of the ''x'' or ''y'' coordinates tends to infinity. In projective geometry and related context ...
s of a single unified mechanism.
Terminology
Förster resonance energy transfer is named after the German scientistTheodor Förster
Theodor Förster (May 15, 1910 – May 20, 1974) was a German physical chemist known for theoretical work on light-matter interaction in molecular systems such as fluorescence and resonant energy transfer.
Education and career
Förster studied ...
. When both chromophores are fluorescent, the term "fluorescence resonance energy transfer" is often used instead, although the energy is not actually transferred by fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
. In order to avoid an erroneous interpretation of the phenomenon that is always a nonradiative transfer of energy (even when occurring between two fluorescent chromophores), the name "Förster resonance energy transfer" is preferred to "fluorescence resonance energy transfer"; however, the latter enjoys common usage in scientific literature. FRET is not restricted to fluorescence and occurs in connection with phosphorescence as well.
Theoretical basis
The FRET efficiency () is thequantum yield The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Applications
Fluorescence spectroscopy
The fluorescence quantum yield is defined as the ratio of the numb ...
of the energy-transfer transition, i.e. the probability of energy-transfer event occurring per donor excitation event:
:
where is the rate of energy transfer, the radiative decay rate of the donor, and the rates of any other de-excitation pathways excluding energy transfers to other acceptors.
The FRET efficiency depends on many physical parameters that can be grouped as: 1) the distance between the donor and the acceptor (typically in the range of 1–10 nm), 2) the spectral overlap of the donor emission spectrum and the acceptor absorption spectrum, and 3) the relative orientation of the donor emission dipole moment and the acceptor absorption dipole moment.
depends on the donor-to-acceptor separation distance with an inverse 6th-power law due to the dipole–dipole coupling mechanism:
:
with being the Förster distance of this pair of donor and acceptor, i.e. the distance at which the energy transfer efficiency is 50%.
The Förster distance depends on the overlap integral
In mathematics, an integral assigns numbers to functions in a way that describes displacement, area, volume, and other concepts that arise by combining infinitesimal data. The process of finding integrals is called integration. Along wit ...
of the donor emission spectrum with the acceptor absorption spectrum and their mutual molecular orientation as expressed by the following equation all in SI units:
:
where is the fluorescence quantum yield The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Applications
Fluorescence spectroscopy
The fluorescence quantum yield is defined as the ratio of the numb ...
of the donor in the absence of the acceptor, is the dipole orientation factor, is the refractive index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.
The refractive index determines how much the path of light is bent, or ...
of the medium, is the Avogadro constant
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining c ...
, and is the spectral overlap integral calculated as
:
where is the donor emission spectrum, is the donor emission spectrum normalized to an area of 1, and is the acceptor molar extinction coefficient
In chemistry, the molar absorption coefficient or molar attenuation coefficient is a measurement of how strongly a chemical species absorbs, and thereby attenuates, light at a given wavelength. It is an intrinsic property of the species. The SI u ...
, normally obtained from an absorption spectrum.
The orientation factor is given by
:
where denotes the normalized transition dipole moment of the respective fluorophore, and denotes the normalized inter-fluorophore displacement.
= 2/3 is often assumed. This value is obtained when both dyes are freely rotating and can be considered to be isotropically oriented during the excited-state lifetime. If either dye is fixed or not free to rotate, then = 2/3 will not be a valid assumption. In most cases, however, even modest reorientation of the dyes results in enough orientational averaging that = 2/3 does not result in a large error in the estimated energy-transfer distance due to the sixth-power dependence of on . Even when is quite different from 2/3, the error can be associated with a shift in , and thus determinations of changes in relative distance for a particular system are still valid. Fluorescent proteins do not reorient on a timescale that is faster than their fluorescence lifetime. In this case 0 ≤ ≤ 4.
The units of the data are usually not in SI units. Using the original units to calculate the Förster distance is often more convenient. For example, the wavelength is often in unit nm and the extinction coefficient is often in unit , where is concentration . obtained from these units will have unit . To use unit Å () for the , the equation is adjusted to
: (Å)
For time-dependent analyses of FRET, the rate of energy transfer () can be used directly instead:
:
where is the donor's fluorescence lifetime in the absence of the acceptor.
The FRET efficiency relates to the quantum yield and the fluorescence lifetime of the donor molecule as follows:
:
where and are the donor fluorescence lifetimes in the presence and absence of an acceptor respectively, or as
:
where and are the donor fluorescence intensities with and without an acceptor respectively.
Experimental confirmation of the Förster resonance energy transfer theory
The inverse sixth-power distance dependence of Förster resonance energy transfer was experimentally confirmed byWilchek
Meir Wilchek (Hebrew: מאיר אשר וילצ'ק, born 17 October 1935) is an Israeli biochemist. He is a professor at the Weizmann Institute of Science.
Early life and education
Meir Wilchek was born in Warsaw, Poland, scion of a rabbinical fa ...
, Edelhoch and Brand using tryptophyl peptides. Stryer, Haugland and Yguerabide also experimentally demonstrated the theoretical dependence of Förster resonance energy transfer on the overlap integral by using a fused indolosteroid as a donor and a ketone as an acceptor. Calculations on FRET distances of some example dye-pairs can be found here.
However, a lot of contradictions of special experiments with the theory was observed under complicated environment when the orientations and quantum yields of the molecules are difficult to estimate.
Methods to measure FRET efficiency
In fluorescence microscopy, fluorescenceconfocal laser scanning microscopy
Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser confocal scanning microscopy (LCSM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a sp ...
, as well as in molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physi ...
, FRET is a useful tool to quantify molecular dynamics in biophysics
Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. ...
and biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
-protein interactions, protein– DNA interactions, and protein conformational changes. For monitoring the complex formation between two molecules, one of them is labeled with a donor and the other with an acceptor. The FRET efficiency is measured and used to identify interactions between the labeled complexes. There are several ways of measuring the FRET efficiency by monitoring changes in the fluorescence emitted by the donor or the acceptor.
Sensitized emission
One method of measuring FRET efficiency is to measure the variation in acceptor emission intensity. When the donor and acceptor are in proximity (1–10 nm) due to the interaction of the two molecules, the acceptor emission will increase because of theintermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
FRET from the donor to the acceptor. For monitoring protein conformational changes, the target protein is labeled with a donor and an acceptor at two loci. When a twist or bend of the protein brings the change in the distance or relative orientation of the donor and acceptor, FRET change is observed. If a molecular interaction or a protein conformational change is dependent on ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
binding, this FRET technique is applicable to fluorescent indicators for the ligand detection.
Photobleaching FRET
FRET efficiencies can also be inferred from thephotobleaching
In optics, photobleaching (sometimes termed fading) is the photochemical alteration of a dye or a fluorophore molecule such that it is permanently unable to fluoresce. This is caused by cleaving of covalent bonds or non-specific reactions between ...
rates of the donor in the presence and absence of an acceptor. This method can be performed on most fluorescence microscopes; one simply shines the excitation light (of a frequency that will excite the donor but not the acceptor significantly) on specimens with and without the acceptor fluorophore and monitors the donor fluorescence (typically separated from acceptor fluorescence using a bandpass filter
A band-pass filter or bandpass filter (BPF) is a device that passes frequencies within a certain range and rejects ( attenuates) frequencies outside that range.
Description
In electronics and signal processing, a filter is usually a two-p ...
) over time. The timescale is that of photobleaching, which is seconds to minutes, with fluorescence in each curve being given by
:
where is the photobleaching decay time constant and depends on whether the acceptor is present or not. Since photobleaching consists in the permanent inactivation of excited fluorophores, resonance energy transfer from an excited donor to an acceptor fluorophore prevents the photobleaching of that donor fluorophore, and thus high FRET efficiency leads to a longer photobleaching decay time constant:
:
where and are the photobleaching decay time constants of the donor in the presence and in the absence of the acceptor respectively. (Notice that the fraction is the reciprocal of that used for lifetime measurements).
This technique was introduced by Jovin in 1989. Its use of an entire curve of points to extract the time constants can give it accuracy advantages over the other methods. Also, the fact that time measurements are over seconds rather than nanoseconds makes it easier than fluorescence lifetime measurements, and because photobleaching decay rates do not generally depend on donor concentration (unless acceptor saturation is an issue), the careful control of concentrations needed for intensity measurements is not needed. It is, however, important to keep the illumination the same for the with- and without-acceptor measurements, as photobleaching increases markedly with more intense incident light.
Lifetime measurements
FRET efficiency can also be determined from the change in the fluorescence lifetime of the donor. The lifetime of the donor will decrease in the presence of the acceptor. Lifetime measurements of the FRET-donor are used influorescence-lifetime imaging microscopy
Fluorescence-lifetime imaging microscopy or FLIM is an imaging technique based on the differences in the exponential decay rate of the photon emission of a fluorophore from a sample. It can be used as an imaging technique in confocal microscopy, t ...
(FLIM).
Single-molecule FRET (smFRET)
Main articlesingle-molecule FRET Single molecule fluorescence (or Förster) resonance energy transfer (or smFRET) is a biophysical technique used to measure distances at the 1-10 nanometer scale in single molecules, typically biomolecules. It is an application of FRET wherein a ...
.
smFRET is a group of methods using various microscopic techniques to measure a pair of donor and acceptor fluorophores that are excited and detected at the single molecule level. In contrast to "ensemble FRET" or "bulk FRET" which provides the FRET signal of a high number of molecules, single-molecule FRET is able to resolve the FRET signal of each individual molecule. The variation of the smFRET signal is useful to reveal kinetic information that an ensemble measurement cannot provide, especially when the system is under equilibrium. Heterogeneity among different molecules can also be observed. This method has been applied in many measurements of biomolecular dynamics such as DNA/RNA/protein folding/unfolding and other conformational changes, and intermolecular dynamics such as reaction, binding, adsorption, and desorption that are particularly useful in chemical sensing, bioassays, and biosensing.
Fluorophores used for FRET

CFP-YFP pairs
One common pair fluorophores for biological use is acyan fluorescent protein
The green fluorescent protein (GFP) is a protein that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range. The label ''GFP'' traditionally refers to the protein first isolated from the jellyfish '' Aequore ...
(CFP) – yellow fluorescent protein
Yellow fluorescent protein (YFP) is a genetic mutant of green fluorescent protein (GFP) originally derived from the jellyfish '' Aequorea victoria''. Its excitation peak is 513 nm and its emission peak is 527 nm. Like the parent GFP, YFP ...
(YFP) pair. Both are color variants of green fluorescent protein (GFP). Labeling with organic fluorescent dyes requires purification, chemical modification, and intracellular injection of a host protein. GFP variants can be attached to a host protein by genetic engineering which can be more convenient. Additionally, a fusion of CFP and YFP ("tandem-dimer") linked by a protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the ...
cleavage sequence can be used as a cleavage assay.
BRET
A limitation of FRET performed with fluorophore donors is the requirement for external illumination to initiate the fluorescence transfer, which can lead to background noise in the results from direct excitation of the acceptor or tophotobleaching
In optics, photobleaching (sometimes termed fading) is the photochemical alteration of a dye or a fluorophore molecule such that it is permanently unable to fluoresce. This is caused by cleaving of covalent bonds or non-specific reactions between ...
. To avoid this drawback, bioluminescence resonance energy transfer (or BRET) has been developed. This technique uses a bioluminescent luciferase (typically the luciferase from ''Renilla reniformis
The sea pansy, ''Renilla reniformis,'' is a species of colonial cnidarian in the family Renillidae, part of an octocoral subclass of Anthozoa that inhabit an expansive range of environments. It is native to warm continental shelf waters of th ...
'') rather than CFP to produce an initial photon emission compatible with YFP.
BRET has also been implemented using a different luciferase enzyme, engineered from the deep-sea shrimp ''Oplophorus gracilirostris''. This luciferase is smaller (19 kD) and brighter than the more commonly used luciferase from ''Renilla reniformis''., and has been named NanoLuc or NanoKAZ. Promega
Promega Corporation is a Madison, Wisconsin-based manufacturer of enzymes and other products for biotechnology and molecular biology with a portfolio covering the fields of genomics, protein analysis and expression, cellular analysis, drug disc ...
has developed a patented substrate for NanoLuc called furimazine, though other valuables coelenterazine substrates for NanoLuc have also been published A split-protein version of NanoLuc has been developed by Promega which has also been used as a BRET donor in experiments measuring protein-protein interactions
Homo-FRET
In general, "FRET" refers to situations where the donor and acceptor proteins (or "fluorophores") are of two different types. In many biological situations, however, researchers might need to examine the interactions between two, or more, proteins of the same type—or indeed the same protein with itself, for example if the protein folds or forms part of a polymer chain of proteins or for other questions of quantification in biological cells. Obviously, spectral differences will not be the tool used to detect and measure FRET, as both the acceptor and donor protein emit light with the same wavelengths. Yet researchers can detect differences in the polarisation between the light which excites the fluorophores and the light which is emitted, in a technique called FRET anisotropy imaging; the level of quantified anisotropy (difference in polarisation between the excitation and emission beams) then becomes an indicative guide to how many FRET events have happened.Others
Various compounds beside fluorescent proteins.Applications
The applications of fluorescence resonance energy transfer (FRET) have expanded tremendously in the last 25 years, and the technique has become a staple in many biological andbiophysical
Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. Bi ...
fields. FRET can be used as a spectroscopic ruler to measure distance and detect molecular interactions in a number of systems and has applications in biology and biochemistry.
Proteins
FRET is often used to detect and track interactions between proteins. Additionally, FRET can be used to measure distances between domains in a single protein by tagging different regions of the protein with fluorophores and measuring emission to determine distance. This provides information aboutprotein conformation
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer ma ...
, including secondary structures
Secondary may refer to: Science and nature
* Secondary emission, of particles
** Secondary electrons, electrons generated as ionization products
* The secondary winding, or the electrical or electronic circuit connected to the secondary winding ...
and protein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
. This extends to tracking functional changes in protein structure, such as conformational changes associated with myosin activity. Applied in vivo, FRET has been used to detect the location and interactions of cellular structures including integrins
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, ...
and membrane protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane ...
s.
Membranes
FRET can be used to observemembrane fluidity
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion ...
, movement and dispersal of membrane proteins, membrane lipid-protein and protein-protein interactions, and successful mixing of different membranes. FRET is also used to study formation and properties of membrane domains and lipid raft
The plasma membranes of cells contain combinations of glycosphingolipids, cholesterol and protein receptors organised in glycolipoprotein lipid microdomains termed lipid rafts. Their existence in cellular membranes remains somewhat controversial ...
s in cell membranes and to determine surface density in membranes.
Chemosensory
 FRET-based probes can detect the presence of various molecules: the probe's structure is affected by small molecule binding or activity, which can turn the FRET system on or off. This is often used to detect anions, cations, small uncharged molecules, and some larger biomacromolecules as well. Similarly, FRET systems have been designed to detect changes in the cellular environment due to such factors as pH, hypoxia, or mitochondrial membrane potential.
FRET-based probes can detect the presence of various molecules: the probe's structure is affected by small molecule binding or activity, which can turn the FRET system on or off. This is often used to detect anions, cations, small uncharged molecules, and some larger biomacromolecules as well. Similarly, FRET systems have been designed to detect changes in the cellular environment due to such factors as pH, hypoxia, or mitochondrial membrane potential.
Signaling pathways
Another use for FRET is in the study of metabolic orsignaling pathways
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellula ...
. For example, FRET and BRET have been used in various experiments to characterize G-protein coupled receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related p ...
activation and consequent signaling mechanisms. Other examples include the use of FRET to analyze such diverse processes as bacterial chemotaxis and caspase activity in apoptosis.
Proteins and nucleotides folding kinetics
Proteins, DNAs, RNAs, and other polymer folding dynamics have been measured using FRET. Usually, these systems are under equilibrium whose kinetics is hidden. However, they can be measured by measuring single-molecule FRET with proper placement of the acceptor and donor dyes on the molecules. Seesingle-molecule FRET Single molecule fluorescence (or Förster) resonance energy transfer (or smFRET) is a biophysical technique used to measure distances at the 1-10 nanometer scale in single molecules, typically biomolecules. It is an application of FRET wherein a ...
for a more detailed description.
Other applications
In addition to common uses previously mentioned, FRET and BRET are also effective in the study of biochemical reaction kinetics. FRET is increasingly used for monitoring pH dependent assembly and disassembly and is valuable in the analysis of nucleic acids encapsulation. This technique can be used to determine factors affecting various types ofnanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
formation as well as the mechanisms and effects of nanomedicine
Nanomedicine is the medical application of nanotechnology. Nanomedicine ranges from the medical applications of nanomaterials and biological devices, to nanoelectronic biosensors, and even possible future applications of molecular nanotech ...
s.
Other methods
A different, but related, mechanism isDexter electron transfer
Dexter electron transfer (also called Dexter electron exchange and Dexter energy transfer) is a fluorescence quenching mechanism in which an excited electron is transferred from one molecule (a donor) to a second molecule (an acceptor) via a n ...
.
An alternative method to detecting protein–protein proximity is the bimolecular fluorescence complementation
Bimolecular fluorescence complementation (also known as BiFC) is a technology typically used to validate protein interactions. It is based on the association of fluorescent protein fragments that are attached to components of the same macromolec ...
(BiFC), where two parts of a fluorescent protein are each fused to other proteins. When these two parts meet, they form a fluorophore on a timescale of minutes or hours.
See also
*Dexter electron transfer
Dexter electron transfer (also called Dexter electron exchange and Dexter energy transfer) is a fluorescence quenching mechanism in which an excited electron is transferred from one molecule (a donor) to a second molecule (an acceptor) via a n ...
* Förster coupling
*Surface energy transfer
Surface energy transfer (SET) is a dipole-surface energy transfer process involving a metallic surface and a molecular dipole.
Formula
The SET rate follows the inverse of the fourth power of the distance
:k_\text = \frac \left(\frac\right)^
where ...
*Time-resolved fluorescence energy transfer Time-resolved fluorescence energy transfer (TR-FRET) is the practical combination of time-resolved fluorometry (TRF) with Förster resonance energy transfer (FRET) that offers a powerful tool for drug discovery researchers. TR-FRET combines the lo ...
References
External links
*FRET Imaging
(Tutorial of Becker & Hickl, website) {{DEFAULTSORT:Forster Resonance Energy Transfer Imaging Fluorescence Biochemistry methods Biophysics Cell imaging Optical phenomena Protein–protein interaction assays Fluorescence techniques Cell biology Laboratory techniques Molecular biology techniques Energy transfer