Fractionation Of Carbon Isotopes In Oxygenic Photosynthesis on:
[Wikipedia]
[Google]
[Amazon]
 Oxygenic
Oxygenic 

 The large fractionation of 13C in photosynthesis is due to the carboxylation reaction, which is carried out by the enzyme ribulose-1,5-bisphosphate carboxylase oxygenase, or
The large fractionation of 13C in photosynthesis is due to the carboxylation reaction, which is carried out by the enzyme ribulose-1,5-bisphosphate carboxylase oxygenase, or
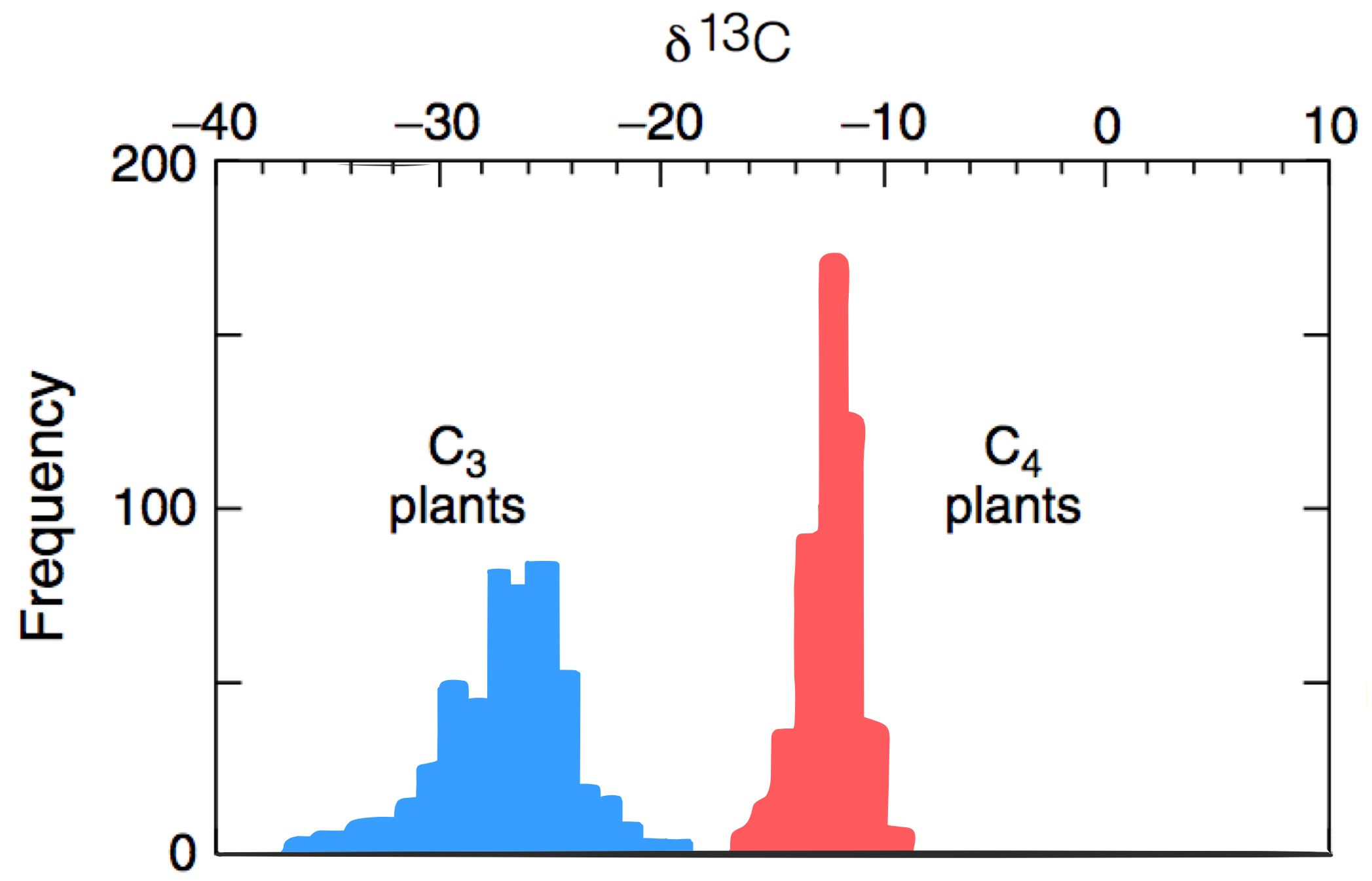 A C3 plant uses
A C3 plant uses
 C4 plants have developed the
C4 plants have developed the
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
converts carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
to carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s via several metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reac ...
s that provide energy to an organism and preferentially react with certain stable isotopes of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
. The selective enrichment of one stable isotope over another creates distinct isotopic fractionations that can be measured and correlated among oxygenic phototroph
Phototrophs () are organisms that carry out photon capture to produce complex organic compounds (e.g. carbohydrates) and acquire energy. They use the energy from light to carry out various cellular metabolic processes. It is a common misconcep ...
s. The degree of carbon isotope fractionation is influenced by several factors, including the metabolism, anatomy, growth rate, and environmental conditions of the organism. Understanding these variations in carbon fractionation across species is useful for biogeochemical
Biogeochemistry is the scientific discipline that involves the study of the chemical, physical, geological, and biological processes and reactions that govern the composition of the natural environment (including the biosphere, the cryosphere, t ...
studies, including the reconstruction of paleoecology
Paleoecology (also spelled palaeoecology) is the study of interactions between organisms and/or interactions between organisms and their environments across geologic timescales. As a discipline, paleoecology interacts with, depends on and informs ...
, plant evolution
Plant evolution is the subset of evolutionary phenomena that concern plants. Evolutionary phenomena are characteristics of populations that are described by averages, medians, distributions, and other statistical methods. This distinguishes pla ...
, and the characterization of food chain
A food chain is a linear network of links in a food web starting from producer organisms (such as grass or algae which produce their own food via photosynthesis) and ending at an apex predator species (like grizzly bears or killer whales), det ...
s.
 Oxygenic
Oxygenic photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
is a metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reac ...
facilitated by autotroph
An autotroph or primary producer is an organism that produces complex organic compounds (such as carbohydrates, fats, and proteins) using carbon from simple substances such as carbon dioxide,Morris, J. et al. (2019). "Biology: How Life Works", ...
s, including plants, algae, and cyanobacteria. This pathway converts inorganic carbon dioxide from the atmosphere or aquatic environment into carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s, using water and energy from light, then releases molecular oxygen as a product. Organic carbon contains less of the stable isotope Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth.
Detection by mass spectrometry
A mass ...
, or 13C, relative to the initial inorganic carbon from the atmosphere or water because photosynthetic carbon fixation involves several fractionating reactions with kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for th ...
s. These reactions undergo a kinetic isotope effect because they are limited by overcoming an activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
barrier. The lighter isotope has a higher energy state
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The te ...
in the quantum well
A quantum well is a potential well with only discrete energy values.
The classic model used to demonstrate a quantum well is to confine particles, which were initially free to move in three dimensions, to two dimensions, by forcing them to occupy ...
of a chemical bond, allowing it to be preferentially formed into products. Different organisms fix carbon through different mechanisms, which are reflected in the varying isotope compositions across photosynthetic pathways (see table below, and explanation of notation in "Carbon Isotope Measurement" section). The following sections will outline the different oxygenic photosynthetic pathways and what contributes to their associated delta values.

Carbon isotope measurement
Carbon on Earth naturally occurs in two stable isotopes, with 98.9% in the form of 12C and 1.1% in 13C. The ratio between these isotopes varies in biological organisms due to metabolic processes that selectively use one carbon isotope over the other, or "fractionate" carbon through kinetic or thermodynamic effects. Oxygenicphotosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
takes place in plants and microorganisms through different chemical pathways, so various forms of organic material reflect different ratios of 13C isotopes. Understanding these variations in carbon fractionation across species is applied in isotope geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal ...
and ecological isotope studies to understand biochemical processes, establish food chains, or model the carbon cycle through geological time.
Carbon isotope fractionations are expressed in using delta notation of ''δ''13C ("delta thirteen C"), which is reported in parts per thousand (per mille
Per mille (from Latin , "in each thousand") is an expression that means parts per thousand. Other recognised spellings include per mil, per mill, permil, permill, or permille.
The associated sign is written , which looks like a percent sig ...
, ‰). ''δ''13C is defined in relation to the Vienna Pee Dee Belemnite
Belemnitida (or the belemnite) is an extinct order of squid-like cephalopods that existed from the Late Triassic to Late Cretaceous. Unlike squid, belemnites had an internal skeleton that made up the cone. The parts are, from the arms-most to ...
(VPDB, 13C/12C = 0.01118) as an established reference standard
A drug reference standard or pharmaceutical reference standard is a highly characterized material suitable to test the identity, strength, quality and purity of substances for pharmaceutical use and medicinal products.
Pharmacopoeial reference s ...
. This is called a "delta value" and can be calculated from the formula below:
Photosynthesis reactions
The chemical pathway of oxygenic photosynthesis fixes carbon in two stages: the light-dependent reactions and the light-independent reactions. The light-dependent reactions capture light energy to transfer electrons from water and convert NADP+, ADP, and inorganic phosphate into the energy-storage moleculesNADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NAD ...
and ATP. The overall equation for the light-dependent reactions is generally:2 H2O + 2 NADP+ + 3 ADP + 3 Pi + light → 2 NADPH + 2 H+ + 3 ATP + O2The light-independent reactions undergo the
Calvin-Benson cycle
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into ...
, in which the energy from NADPH and ATP is used to convert carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and water into organic compounds via the enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
RuBisCO
Ribulose-1,5-bisphosphate carboxylase-oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme () involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is con ...
.
The overall general equation for the light-independent reactions is the following:3 CO2 + 9 ATP + 6 NADPH + 6 H+ → C3H6O3-phosphate + 9 ADP + 8 Pi + 6 NADP+ + 3 H2OThe 3-carbon products (C3H6O3-phosphate) of the Calvin cycle are later converted to
glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
or other carbohydrates such as starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets ...
, sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
, and cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
.
Fractionation via RuBisCO
RuBisCO
Ribulose-1,5-bisphosphate carboxylase-oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme () involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is con ...
. RuBisCO catalyzes the reaction between a five-carbon molecule, ribulose-1,5-bisphosphate
Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal acceptor in plants. It is a colourless anion, a double phosphate ester of the ketopentose (ketone-containing sugar with five car ...
(abbreviated as RuBP) and CO2 to form two molecules of 3-phosphoglyceric acid (abbreviated as PGA). PGA reacts with NADPH to produce 3-phosphoglyceraldehyde.
Isotope fractionation due to Rubisco (form I) carboxylation alone is predicted to be a 28‰ depletion, on average. However, fractionation values vary between organisms, ranging from an 11‰ depletion observed in coccolithophorid
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingd ...
algae to a 29‰ depletion observed in spinach
Spinach (''Spinacia oleracea'') is a leafy green flowering plant native to central and western Asia. It is of the order Caryophyllales, family Amaranthaceae, subfamily Chenopodioideae. Its leaves are a common edible vegetable consumed either f ...
. RuBisCO causes a kinetic isotope effect because 12CO2 and 13CO2 compete for the same active site and 13C has an intrinsically lower reaction rate.
13C fractionation model
In addition to the discriminating effects of enzymatic reactions, the diffusion of CO2 gas to the carboxylation site within a plant cell also influences isotopic fractionation. Depending on the type of plant (see sections below), external CO2 must be transported through theboundary layer
In physics and fluid mechanics, a boundary layer is the thin layer of fluid in the immediate vicinity of a bounding surface formed by the fluid flowing along the surface. The fluid's interaction with the wall induces a no-slip boundary condi ...
and stomata
In botany, a stoma (from Greek ''στόμα'', "mouth", plural "stomata"), also called a stomate (plural "stomates"), is a pore found in the epidermis of leaves, stems, and other organs, that controls the rate of gas exchange. The pore is bor ...
and into the internal gas space of a plant cell, where it dissolves and diffuses to the chloroplast. The diffusivity
Diffusivity is a rate of diffusion, a measure of the rate at which particles or heat or fluids can spread.
It is measured differently for different mediums.
Diffusivity may refer to:
*Thermal diffusivity, diffusivity of heat
*Diffusivity of mass: ...
of a gas is inversely proportional to the square root of its molecular reduced mass
In physics, the reduced mass is the "effective" Mass#Inertial mass, inertial mass appearing in the two-body problem of Newtonian mechanics. It is a quantity which allows the two-body problem to be solved as if it were a one-body problem. Note, how ...
(relatively to air), causing 13CO2 to be 4.4‰ less diffusive than 12CO2.
A prevailing model for fractionation of atmospheric CO2 in plants combines the isotope effects of the carboxylation reaction with the isotope effects from gas diffusion into the plant in the following equation:
Where:
* δ13Csample is the delta-value of the organism for 13C composition
*δ13Catm is the delta-value of atmospheric CO2, which is = -7.8‰
* the discrimination due to diffusion ''a'' = 4.4‰
* the carboxylation discrimination ''b'' = 30‰
* ca is the partial pressure of CO2 in the external atmosphere, and
* ci is the partial pressure of CO2 in the intercellular spaces.
This model, derived ''ab initio
''Ab initio'' ( ) is a Latin term meaning "from the beginning" and is derived from the Latin ''ab'' ("from") + ''initio'', ablative singular of ''initium'' ("beginning").
Etymology
Circa 1600, from Latin, literally "from the beginning", from ab ...
'', generally describes fractionation of carbon in the majority of plants, which facilitate C3 carbon fixation
carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, along with and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosph ...
. Modifications have been made to this model with empirical findings. However, several additional factors, not included in this general model, will increase or decrease 13C fractionation across species. Such factors include the competing oxygenation reaction of RuBisCO, anatomical and temporal adaptations to enzyme activity, and variations in cell growth and geometry. The isotopic fractionations of different photosynthetic pathways are uniquely characterized by these factors, as described below.
In C3 plants
 A C3 plant uses
A C3 plant uses C3 carbon fixation
carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, along with and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosph ...
, one of the three metabolic photosynthesis pathways which also include C4 and CAM
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the bin ...
(described below). These plants are called "C3" due to the three-carbon compound ( 3-Phosphoglyceric acid, or 3-PGA) produced by the CO2 fixation mechanism in these plants. This C3 mechanism is the first step of the Calvin-Benson cycle, which converts CO2 and RuBP
Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal acceptor in plants. It is a colourless anion, a double phosphate ester of the ketopentose ( ketone-containing sugar with five c ...
into 3-PGA.
C3 plants are the most common type of plant, and typically thrive under moderate sunlight intensity and temperatures, CO2 concentrations above 200 ppm, and abundant groundwater. C3 plants do not grow well in very hot or arid regions, in which C4 and CAM plants are better adapted.
The isotope fractionations in C3 carbon fixation arise from the combined effects of CO2 gas diffusion through the stomata
In botany, a stoma (from Greek ''στόμα'', "mouth", plural "stomata"), also called a stomate (plural "stomates"), is a pore found in the epidermis of leaves, stems, and other organs, that controls the rate of gas exchange. The pore is bor ...
of the plant, and the carboxylation via RuBisCO
Ribulose-1,5-bisphosphate carboxylase-oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme () involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is con ...
. Stomatal conductance Stomatal conductance, usually measured in mmol m−2 s−1 by a porometer, estimates the rate of gas exchange (i.e., carbon dioxide uptake) and transpiration (i.e., water loss as water vapor) through the leaf stomata as determined by the degree of ...
discriminates against the heavier 13C by 4.4‰. RuBisCO carboxylation contributes a larger discrimination of 27‰.
RuBisCO enzyme catalyzes the carboxylation of CO2 and the 5-carbon sugar, RuBP
Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal acceptor in plants. It is a colourless anion, a double phosphate ester of the ketopentose ( ketone-containing sugar with five c ...
, into 3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. Th ...
, a 3-carbon compound through the following reaction:
The product 3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. Th ...
is depleted in 13C due to the kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for th ...
of the above reaction. The overall 13C fractionation for C3 photosynthesis ranges between -20 to -37‰.
The wide range of variation in delta values expressed in C3 plants is modulated by the stomatal conductance Stomatal conductance, usually measured in mmol m−2 s−1 by a porometer, estimates the rate of gas exchange (i.e., carbon dioxide uptake) and transpiration (i.e., water loss as water vapor) through the leaf stomata as determined by the degree of ...
, or the rate of CO2 entering, or water vapor exiting, the small pores in the epidermis of a leaf. The δ13C of C3 plants depends on the relationship between stomatal conductance and photosynthetic rate, which is a good proxy of water use efficiency in the leaf. C3 plants with high water-use efficiency tend to be less fractionated in 13C (i.e., δ13C is relatively less negative) compared to C3 plants with low water-use efficiency.
In C4 plants
C4 carbon fixation
carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960's discovery by Marshall Davidson Hatch and Charles Roger Slack that some plants, when sup ...
pathway to conserve water loss, thus are more prevalent in hot, sunny, and dry climates. These plants differ from C3 plants because CO2 is initially converted to a four-carbon molecule, malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L ...
, which is shuttled to bundle sheath cells, released back as CO2 and only then enters the Calvin Cycle. In contrast, C3 plants directly perform the Calvin Cycle in mesophyll cells, without making use of a CO2 concentration method. Malate, the four-carbon compound is the namesake of "C4" photosynthesis. This pathway allows C4 photosynthesis to efficiently shuttle CO2 to the RuBisCO enzyme and maintain high concentrations of CO2 within bundle sheath cells. These cells are part of the characteristic ''kranz leaf anatomy'', which spatially separates photosynthetic cell-types in a concentric arrangement to accumulate CO2 near RuBisCO.
These chemical and anatomical mechanisms improve the ability of RuBisCO to fix carbon, rather than perform its wasteful oxygenase
An oxygenase is any enzyme that oxidizes a substrate by transferring the oxygen from molecular oxygen O2 (as in air) to it. The oxygenases form a class of oxidoreductases; their EC number is EC 1.13 or EC 1.14.
Discoverers
Oxygenases were disco ...
activity. The RuBisCO oxygenase activity, called photorespiration
Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. The desired reaction i ...
, causes the RuBP substrate to be lost to oxygenation, and consumes energy in doing so. The adaptations of C4 plants provide an advantage over the C3 pathway, which loses efficiency due to photorespiration. The ratio of photorespiration to photosynthesis in a plant varies with environmental conditions, since decreased CO2 and elevated O2 concentrations would increase the efficiency of photorespiration. Atmospheric CO2 on Earth decreased abruptly at a point between 32 and 25 million years ago. This gave a selective advantage to the evolution of the C4 pathway, which can limit photorespiration rate despite the reduced ambient CO2. Today, C4 plants represent roughly 5% of plant biomass on Earth, but about 23% of terrestrial carbon fixation. Types of plants which use C4 photosynthesis include grasses
Poaceae () or Gramineae () is a large and nearly ubiquitous family of monocotyledonous flowering plants commonly known as grasses. It includes the cereal grasses, bamboos and the grasses of natural grassland and species cultivated in lawns and ...
and economically important crops, such as maize
Maize ( ; ''Zea mays'' subsp. ''mays'', from es, maíz after tnq, mahiz), also known as corn (North American and Australian English), is a cereal grain first domesticated by indigenous peoples in southern Mexico about 10,000 years ago. Th ...
, sugar cane
Sugarcane or sugar cane is a species of (often hybrid) tall, perennial grass (in the genus ''Saccharum'', tribe Andropogoneae) that is used for sugar production. The plants are 2–6 m (6–20 ft) tall with stout, jointed, fibrous stalks t ...
, millet
Millets () are a highly varied group of small-seeded grasses, widely grown around the world as cereal crops or grains for fodder and human food. Most species generally referred to as millets belong to the tribe Paniceae, but some millets al ...
, and sorghum
''Sorghum'' () is a genus of about 25 species of flowering plants in the grass family (Poaceae). Some of these species are grown as cereals for human consumption and some in pastures for animals. One species is grown for grain, while many othe ...
.
Isotopic fractionation differs between C4 carbon fixation
carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960's discovery by Marshall Davidson Hatch and Charles Roger Slack that some plants, when sup ...
and C3, due to the spatial separation in C4 plants of CO2 capture (in the mesophyll cells) and the Calvin cycle (in the bundle sheath cells). In C4 plants, carbon is converted to bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
, fixed into oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
via the enzyme phosphoenolpyruvate (PEP) carboxylase, and is then converted to malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L ...
. The malate is transported from the mesophyll
A leaf ( : leaves) is any of the principal appendages of a vascular plant stem, usually borne laterally aboveground and specialized for photosynthesis. Leaves are collectively called foliage, as in "autumn foliage", while the leaves, ste ...
to bundle sheath
A vascular bundle is a part of the transport system in vascular plants. The transport itself happens in the stem, which exists in two forms: xylem and phloem. Both these tissues are present in a vascular bundle, which in addition will inclu ...
cells, which are impermeable to CO2. The internal CO2 is concentrated in these cells as malate is reoxidized then decarboxylated back into CO2 and pyruvate. This enables RuBisCO to perform catalysis while internal CO2 is sufficiently high to avoid the competing photorespiration reaction. The delta value in the C4 pathway is -12 to -16‰ depleted in 13C due to the combined effects of PEP carboxylase and RuBisCO.
The isotopic discrimination in the C4 pathway varies relative to the C3 pathway due to the additional chemical conversion steps and activity of PEP carboxylase. After diffusion into the stomata, the conversion of CO2 to bicarbonate concentrates the heavier 13C. The subsequent fixation via PEP carboxylase is thereby less depleted in 13C than that from Rubisco: about 2‰ depleted in PEP carboxylase, versus 29‰ in RuBisCO. However, a portion of the isotopically heavy carbon that is fixed by PEP carboxylase leaks out of the bundle sheath cells. This limits the carbon available to RuBisCO, which in turn lowers its fractionation effect. This accounts for the overall delta value in C4 plants to be -12 to -16 ‰.
In CAM plants
Plants that useCrassulacean acid metabolism
Crassulacean acid metabolism, also known as CAM photosynthesis, is a carbon fixation pathway that evolved in some plants as an adaptation to arid conditions that allows a plant to photosynthesize during the day, but only exchange gases at night. ...
, also known as CAM photosynthesis, temporally separate their chemical reactions between day and night. This strategy modulates stomatal conductance to increase water-use efficiency, so is well-adapted for arid climates. During the night, CAM plants open stomata to allow CO2 to enter the cell and undergo fixation into organic acids that are stored in vacuoles. This carbon is released to the Calvin cycle during the day, when stomata are closed to prevent water loss, and the light reactions can drive the necessary ATP and NADPH production. This pathway differs from C4 photosynthesis because CAM plants separate carbon by storing fixed CO2 in vesicles at night, then transporting it for use during the day. Thus, CAM plants temporally concentrate CO2 to improve RuBisCO efficiency, whereas C4 plants spatially concentrate CO2 in bundle sheath cells. The distribution of plants which use CAM photosynthesis includes epiphyte
An epiphyte is an organism that grows on the surface of a plant and derives its moisture and nutrients from the air, rain, water (in marine environments) or from debris accumulating around it. The plants on which epiphytes grow are called phoroph ...
s (e.g., orchids
Orchids are plants that belong to the family Orchidaceae (), a diverse and widespread group of flowering plants with blooms that are often colourful and fragrant.
Along with the Asteraceae, they are one of the two largest families of flowering ...
, bromeliads
The Bromeliaceae (the bromeliads) are a family of monocot flowering plants of about 80 genera and 3700 known species, native mainly to the tropical Americas, with several species found in the American subtropics and one in tropical west Africa, ...
) and xerophyte
A xerophyte (from Ancient Greek language, Greek ξηρός ''xeros'' 'dry' + φυτόν ''phuton'' 'plant') is a species of plant that has adaptations to survive in an environment with little liquid water, such as a desert such as the Sahara or pl ...
s (e.g., succulents
In botany, succulent plants, also known as succulents, are plants with parts that are thickened, fleshy, and engorged, usually to retain water in arid climates or soil conditions. The word ''succulent'' comes from the Latin word ''sucus'', meani ...
, cacti
A cactus (, or less commonly, cactus) is a member of the plant family Cactaceae, a family comprising about 127 genera with some 1750 known species of the order Caryophyllales. The word ''cactus'' derives, through Latin, from the Ancient Greek ...
).
In Crassulacean acid metabolism, isotopic fractionation combines the effects of the C3 pathway in the daytime and the C4 pathway in the nighttime. At night, when temperature and water loss are lower, the CO2 diffuses through the stomata and produce malate via phosphenolpyruvate carboxylase. During the following day, stomata are closed, malate is decarboxylated, and CO2 is fixed by RuBisCO. This process alone is similar to that of C4 plants and yields characteristic C4 fractionation values of approximately -11‰. However, in the afternoon, CAM plants may open their stomata and perform C3 photosynthesis. In daytime alone, CAM plants have approximately -28‰ fractionation, characteristic of C3 plants. These combined effects provide ''δ''13C values for CAM plants in the range of -10 to -20‰.
The 13C to 12C ratio in CAM plants can indicate the temporal separation of CO2 fixation, which is the extent of biomass derived from nocturnal CO2 fixation relative to diurnal CO2 fixation. This distinction can be made because PEP carboxylase, the enzyme responsible for net CO2 uptake at night, discriminates 13C less than RuBisCO, which is responsible to daytime CO2 uptake. CAM plants which fix CO2 primarily at night would be predicted to show ''δ''13C values more similar to C4 plants, whereas daytime CO2 fixation would show ''δ''13C values more similar to C3 plants.
In phytoplankton
In contrast to terrestrial plants, where CO2 diffusion in air is relatively fast and typically not limiting, diffusion of dissolved CO2 in water is considerably slower and can often limit carbon fixation in phytoplankton. As gaseous CO2(g) is dissolved into aqueous CO2(aq), it is fractionated by both kinetic and equilibrium effects that are temperature-dependent. Relative to plants, the dissolved CO2 source for phytoplankton can be enriched in 13C by about 8‰ from atmospheric CO2. Isotope fractionation of 13C byphytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words (), meaning 'plant', and (), meaning 'wanderer' or 'drifter'.
Ph ...
photosynthesis is affected by the diffusion of extracellular aqueous CO2 into the cell, the RuBisCO-dependent cell growth rate, and the cell geometry and surface area. The use of bicarbonate and carbon-concentrating mechanisms in phytoplankton distinguishes the isotopic fractionation from plant photosynthetic pathways.
The difference between intracellular and extracellular CO2 concentrations reflects the CO2 demand of a phytoplankton cell, which is dependent on its growth rate. The ratio of carbon demand to supply governs the diffusion of CO2 into the cell, and is negatively correlated with the magnitude of the carbon fractionation by phytoplankton. Combined, these relationships allow the fractionation between CO2(aq) and phytoplankton biomass to be used to estimate the phytoplankton growth rates.
However, growth rate alone does not account for observed fractionation. The flux of CO2(aq) into and out of a cell is roughly proportional to the cell surface area, and the cell carbon biomass varies as a function of cell volume. Phytoplankton geometry that maximizes surface area to volume should have larger isotopic fractionation from photosynthesis.
The biochemical characteristics of phytoplankton are similar to C3 plants, whereas the gas exchange characteristics more closely resemble the C4 strategy. More specifically, phytoplankton improve the efficiency of their primary carbon-fixing enzyme, RuBisCO, with carbon concentrating mechanisms (CCM), just as C4 plants accumulate CO2 in the bundle sheath cells. Different forms of CCM in phytoplankton include the active uptake of bicarbonate and CO2 through the cell membrane, the active transport
In cellular biology, ''active transport'' is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellul ...
of inorganic carbon from the cellular membrane to the chloroplasts, and active, unidirectional conversion of CO2 to bicarbonate. The parameters affecting 13C fractionation in phytoplankton contribute to ''δ''13C values between -18 to -25‰.
See also
*RuBisCO
Ribulose-1,5-bisphosphate carboxylase-oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme () involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is con ...
*Isotope geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal ...
* Intrinsic KIE of RuBisCO
* Paleoclimatology
Paleoclimatology (British spelling, palaeoclimatology) is the study of climates for which direct measurements were not taken. As instrumental records only span a tiny part of Earth's history, the reconstruction of ancient climate is important to ...
References
{{Reflist Isotope separation Isotopes of carbon