Ferromagnetic materials on:
[Wikipedia]
[Google]
[Amazon]
 Ferromagnetism is a property of certain materials (such as
Ferromagnetism is a property of certain materials (such as
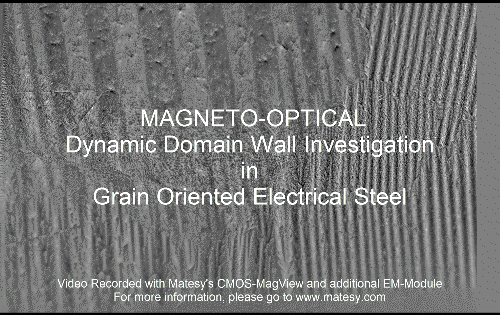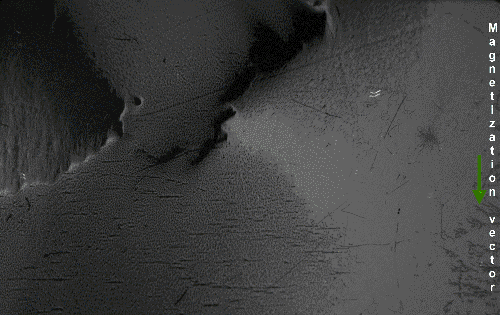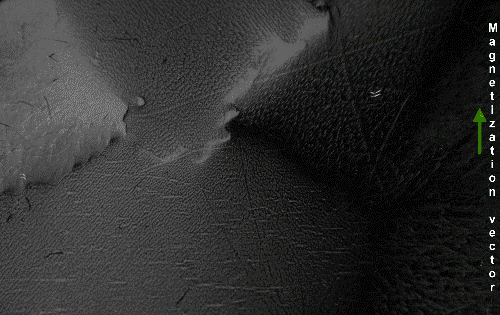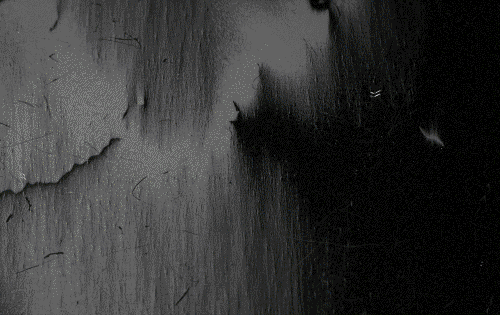
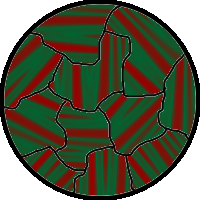 The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called ''
The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called ''
 Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the
Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the
Electromagnetism
– ch. 11, from an online textbook * Detailed nonmathematical description of ferromagnetic materials with illustrations
Magnetism: Models and Mechanisms
in E. Pavarini, E. Koch, and U. Schollwöck: Emergent Phenomena in Correlated Matter, Jülich 2013, {{Authority control Quantum phases Magnetic hysteresis Physical phenomena Ferromagnetism
 Ferromagnetism is a property of certain materials (such as
Ferromagnetism is a property of certain materials (such as iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
) which results in a large observed magnetic permeability
In electromagnetism, permeability is the measure of magnetization that a material obtains in response to an applied magnetic field. Permeability is typically represented by the (italicized) Greek letter ''μ''. The term was coined by William ...
, and in many cases a large magnetic coercivity
Coercivity, also called the magnetic coercivity, coercive field or coercive force, is a measure of the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. Coercivity is usually measured i ...
allowing the material to form a permanent magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, ...
. Ferromagnetic materials are the familiar metals noticeably attracted to a magnet, a consequence of their large magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an ''external'' magnetic field, and it is this temporarily induced magnetization inside a steel plate, for instance, which accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself, depends not only on the strength of the applied field, but on the so-called coercivity of that material, which varies greatly among ferromagnetic materials.
In physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which r ...
, several different types of material magnetism
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particles ...
are distinguished. Ferromagnetism (along with the similar effect ferrimagnetism
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
) is the strongest type and is responsible for the common phenomenon of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism—paramagnetism
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
, diamagnetism
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracte ...
, and antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
—but the forces are usually so weak that they can be detected only by sensitive instruments in a laboratory. An everyday example of a permanent magnet formed of a ferromagnetic material is a refrigerator magnet
A refrigerator magnet or fridge magnet is a small magnet, often attached to an artistic or whimsical ornament, which may be used to post items such as shopping lists, Christmas cards, child art or reminders on a refrigerator door, or which sim ...
used to hold notes on a refrigerator door. The attraction between a magnet and a ferromagnetic material like iron has been described as "the quality of magnetism first apparent to the ancient world, and to us today".Bozorth, Richard M. ''Ferromagnetism'', first published 1951, reprinted 1993 by IEEE
The Institute of Electrical and Electronics Engineers (IEEE) is a 501(c)(3) professional association for electronic engineering and electrical engineering (and associated disciplines) with its corporate office in New York City and its operation ...
Press, New York as a "Classic Reissue". .
Permanent magnets (materials that can be magnetized by an external magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are the materials that are noticeably attracted to them. Relatively few materials are ferromagnetic, and usually are pure forms, alloys, or compounds of iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
, and certain rare-earth metals
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silve ...
. Beyond its chemical composition, a material's ferromagnetic properties (or lack thereof) is affected by its crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
. Ferromagnetism is very important in industry and modern technology and is the basis for many electrical and electromechanical devices such as electromagnet
An electromagnet is a type of magnet in which the magnetic field is produced by an electric current. Electromagnets usually consist of wire wound into a coil. A current through the wire creates a magnetic field which is concentrated in the ...
s, electric motor
An electric motor is an Electric machine, electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a Electromagneti ...
s, generators, transformer
A transformer is a passive component that transfers electrical energy from one electrical circuit to another circuit, or multiple circuits. A varying current in any coil of the transformer produces a varying magnetic flux in the transformer' ...
s, and magnetic storage
Magnetic storage or magnetic recording is the storage of data on a magnetized medium. Magnetic storage uses different patterns of magnetisation in a magnetizable material to store data and is a form of non-volatile memory. The information is acc ...
such as tape recorder
An audio tape recorder, also known as a tape deck, tape player or tape machine or simply a tape recorder, is a sound recording and reproduction device that records and plays back sounds usually using magnetic tape for storage. In its present- ...
s, and hard disk
A hard disk drive (HDD), hard disk, hard drive, or fixed disk is an electro-mechanical data storage device that stores and retrieves digital data using magnetic storage with one or more rigid rapidly rotating platters coated with magnet ...
s, and nondestructive testing of ferrous materials.
Ferromagnetic materials can be divided into magnetically "soft" materials like annealed iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
, which can be magnetized but do not tend to stay magnetized, and magnetically "hard" materials, which do. Permanent magnets are made from "hard" ferromagnetic materials such as alnico
Alnico is a family of iron alloys which in addition to iron are composed primarily of aluminium (Al), nickel (Ni), and cobalt (Co), hence the acronym ''al-ni-co''. They also include copper, and sometimes titanium. Alnico alloys are ferromagnetic, ...
and ferrimagnetic materials such as ferrite that are subjected to special processing in a strong magnetic field during manufacture to align their internal microcrystalline
A microcrystalline material is a crystallized substance or rock that contains small crystals visible only through microscopic examination. There is little agreement on the range of crystal sizes that should be regarded as microcrystalline, but th ...
structure, making them very hard to demagnetize. To demagnetize a saturated magnet, a certain magnetic field must be applied, and this threshold depends on coercivity
Coercivity, also called the magnetic coercivity, coercive field or coercive force, is a measure of the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. Coercivity is usually measured in ...
of the respective material. "Hard" materials have high coercivity, whereas "soft" materials have low coercivity. The overall strength of a magnet is measured by its magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets ...
or, alternatively, the total magnetic flux
In physics, specifically electromagnetism, the magnetic flux through a surface is the surface integral of the normal component of the magnetic field B over that surface. It is usually denoted or . The SI unit of magnetic flux is the weber ( ...
it produces. The local strength of magnetism in a material is measured by its magnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Di ...
.
History and distinction from ferrimagnetism
Historically, the term ''ferromagnetism'' was used for any material that could exhibit spontaneousmagnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Di ...
: a net magnetic moment in the absence of an external magnetic field; that is any material that could become a magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, ...
. This general definition is still in common use.
However, in a landmark paper in 1948, Louis Néel
Louis Eugène Félix Néel (22 November 1904 – 17 November 2000) was a French physicist born in Lyon who received the Nobel Prize for Physics in 1970 for his studies of the magnetic properties of solids.
Biography
Néel studied at the Lycé ...
showed that there are two levels of magnetic alignment that result in this behavior. One is ferromagnetism in the strict sense, where all the magnetic moments are aligned. The other is ''ferrimagnetism
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
'', where some magnetic moments point in the opposite direction but have a smaller contribution, so there is still a spontaneous magnetization.
In the special case where the opposing moments balance completely, the alignment is known as ''antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
''. Therefore antiferromagnets do not have a spontaneous magnetization.
Ferromagnetic materials
Ferromagnetism is an unusual property that occurs in only a few substances. The common ones are thetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
and their alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, ...
s, and alloys of rare-earth metal
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous sil ...
s. It is a property not just of the chemical make-up of a material, but of its crystalline structure and microstructure. Their ferromagnetism results from having many unpaired electrons in their d-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
in the case of iron and its relatives, or the f-block in the case of the rare-earth metals, a result of Hund's rule of maximum multiplicity
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configurati ...
. There are ferromagnetic metal alloys whose constituents are not themselves ferromagnetic, called Heusler alloy
Heusler compounds are magnetic intermetallics with face-centered cubic crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metals and Z is in the p-block. The term derives from the name ...
s, named after Fritz Heusler
Carl Ludwig David Friedrich Heusler (1 February 1866, Dillenburg – 25 October 1947) was a German mining engineer and chemist. He discovered a special group of intermetallics now known as Heusler alloy, Heusler phases, which are ferromagnetic ...
. Conversely, there are non-magnetic alloys, such as types of stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
, composed almost exclusively of ferromagnetic metals.
Amorphous (non-crystalline) ferromagnetic metallic alloys can be made by very rapid quenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as pha ...
(cooling) of a liquid alloy. These have the advantage that their properties are nearly isotropic (not aligned along a crystal axis); this results in low coercivity
Coercivity, also called the magnetic coercivity, coercive field or coercive force, is a measure of the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. Coercivity is usually measured in ...
, low hysteresis
Hysteresis is the dependence of the state of a system on its history. For example, a magnet may have more than one possible magnetic moment in a given magnetic field, depending on how the field changed in the past. Plots of a single component of ...
loss, high permeability, and high electrical resistivity. One such typical material is a transition metal-metalloid
A metalloid is a type of chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on ...
alloy, made from about 80% transition metal (usually Fe, Co, or Ni) and a metalloid component ( B, C, Si, P, or Al) that lowers the melting point.
A relatively new class of exceptionally strong ferromagnetic materials are the rare-earth magnets. They contain lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
elements that are known for their ability to carry large magnetic moments in well-localized f-orbitals.
The table lists a selection of ferromagnetic and ferrimagnetic compounds, along with the temperature above which they cease to exhibit spontaneous magnetization (see Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
).
Unusual materials
Most ferromagnetic materials are metals, since the conducting electrons are often responsible for mediating the ferromagnetic interactions. It is therefore a challenge to develop ferromagnetic insulators, especiallymultiferroic Multiferroics are defined as materials that exhibit more than one of the primary ferroic properties in the same phase:
* ferromagnetism – a magnetisation that is switchable by an applied magnetic field
* ferroelectricity – an electric polarisa ...
materials, which are both ferromagnetic and ferroelectric
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoelectric and pyroelectric, with the ad ...
.
A number of actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
compounds are ferromagnets at room temperature or exhibit ferromagnetism upon cooling. Pu P is a paramagnet with cubic symmetry at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
, but which undergoes a structural transition into a tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square ...
state with ferromagnetic order when cooled below its ''T''C = 125 K. In its ferromagnetic state, PuP's easy axis
In condensed matter physics, magnetic anisotropy describes how an object's magnetic properties can be different depending on direction. In the simplest case, there is no preferential direction for an object's magnetic moment. It will respond to ...
is in the ⟨100⟩ direction.
In NpFe2 the easy axis is ⟨111⟩. Above , NpFe2 is also paramagnetic and cubic. Cooling below the Curie temperature produces a rhombohedral
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a three-dimensional figure with six faces which are rhombi. It is a special case of a parallelepiped where all edges are the same length. It can be us ...
distortion wherein the rhombohedral angle changes from 60° (cubic phase) to 60.53°. An alternate description of this distortion is to consider the length ''c'' along the unique trigonal axis (after the distortion has begun) and ''a'' as the distance in the plane perpendicular to ''c''. In the cubic phase this reduces to . Below the Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
:
which is the largest strain in any actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
compound. NpNi2 undergoes a similar lattice distortion below , with a strain of (43 ± 5) × 10−4. NpCo2 is a ferrimagnet below 15 K.
In 2009, a team of MIT
The Massachusetts Institute of Technology (MIT) is a private land-grant research university in Cambridge, Massachusetts. Established in 1861, MIT has played a key role in the development of modern technology and science, and is one of the m ...
physicists demonstrated that a lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
gas cooled to less than one kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phys ...
can exhibit ferromagnetism. The team cooled fermion
In particle physics, a fermion is a particle that follows Fermi–Dirac statistics. Generally, it has a half-odd-integer spin: spin , spin , etc. In addition, these particles obey the Pauli exclusion principle. Fermions include all quarks an ...
ic lithium-6
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 and lithium-7, with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon ( for ...
to less than (150 billionths of one kelvin) using infrared laser cooling
Laser cooling includes a number of techniques in which atoms, molecules, and small mechanical systems are cooled, often approaching temperatures near absolute zero. Laser cooling techniques rely on the fact that when an object (usually an atom) ...
. This demonstration is the first time that ferromagnetism has been demonstrated in a gas.
In 2018, a team of University of Minnesota
The University of Minnesota, formally the University of Minnesota, Twin Cities, (UMN Twin Cities, the U of M, or Minnesota) is a public university, public Land-grant university, land-grant research university in the Minneapolis–Saint Paul, Tw ...
physicists demonstrated that body-centered tetragonal ruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to ...
exhibits ferromagnetism at room temperature.
Electrically induced ferromagnetism
Recent research has shown evidence that ferromagnetism can be induced in some materials by an electric current or voltage. Antiferromagnetic LaMnO3 and SrCoO have been switched to ferromagnetic by a current. In July 2020 scientists reported inducing ferromagnetism in the abundantdiamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
material iron pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue giv ...
("fool's gold") by an applied voltage. In these experiments the ferromagnetism was limited to a thin surface layer.
Explanation
The Bohr–Van Leeuwen theorem, discovered in the 1910s, showed thatclassical physics
Classical physics is a group of physics theories that predate modern, more complete, or more widely applicable theories. If a currently accepted theory is considered to be modern, and its introduction represented a major paradigm shift, then the ...
theories are unable to account for any form of material magnetism, including ferromagnetism; the explanation rather depends on the quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
description of atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s. Each of an atom's electrons has a magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets ...
according to its spin state, as described by quantum mechanics. The Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated ...
, also a consequence of quantum mechanics, restricts the occupancy of electrons' spin states in atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
, generally causing the magnetic moments from an atom's electrons to largely or completely cancel. An atom will have a ''net'' magnetic moment when that cancellation is incomplete.
Origin of atomic magnetism
One of the fundamental properties of anelectron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
(besides that it carries charge) is that it has a magnetic dipole moment, i.e., it behaves like a tiny magnet, producing a magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
. This dipole moment comes from the more fundamental property of the electron that it has quantum mechanical spin. Due to its quantum nature, the spin of the electron can be in one of only two states; with the magnetic field either pointing "up" or "down" (for any choice of up and down). The spin of the electrons in atoms is the main source of ferromagnetism, although there is also a contribution from the orbital angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syst ...
of the electron about the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
* Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
. When these magnetic dipoles in a piece of matter are aligned, (point in the same direction) their individually tiny magnetic fields add together to create a much larger macroscopic field.
However, materials made of atoms with filled electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
s have a total dipole moment of zero: because the electrons all exist in pairs with opposite spin, every electron's magnetic moment is cancelled by the opposite moment of the second electron in the pair. Only atoms with partially filled shells (i.e., unpaired spins) can have a net magnetic moment, so ferromagnetism occurs only in materials with partially filled shells. Because of Hund's rules
In atomic physics, Hund's rules refers to a set of rules that German physicist Friedrich Hund formulated around 1927, which are used to determine the term symbol that corresponds to the ground state of a multi-electron atom. The first rule is e ...
, the first few electrons in a shell tend to have the same spin, thereby increasing the total dipole moment.
These unpaired dipoles (often called simply "spins", even though they also generally include orbital angular momentum) tend to align in parallel to an external magnetic field leading to a macroscopic effect called paramagnetism
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
. In ferromagnetism, however, the magnetic interaction between neighboring atoms' magnetic dipoles is strong enough that they align with ''each other'' regardless of any applied field, resulting in the spontaneous magnetization
Spontaneous magnetization is the appearance of an ordered spin state ( magnetization) at zero applied magnetic field in a ferromagnetic or ferrimagnetic material below a critical point called the Curie temperature or .
Overview
Heated to tempe ...
of so-called domains. This results in the large observed magnetic permeability
In electromagnetism, permeability is the measure of magnetization that a material obtains in response to an applied magnetic field. Permeability is typically represented by the (italicized) Greek letter ''μ''. The term was coined by William ...
of ferromagnetics, and the ability of "hard" magnetic materials to form permanent magnets
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, ...
.
Exchange interaction
When two nearby atoms have unpaired electrons, whether the electron spins are parallel or antiparallel affects whether the electrons can share the same orbit as a result of thequantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
effect called the exchange interaction
In chemistry and physics, the exchange interaction (with an exchange energy and exchange term) is a quantum mechanical effect that only occurs between identical particles. Despite sometimes being called an exchange force in an analogy to classical ...
. This in turn affects the electron location and the Coulomb (electrostatic) interaction and thus the energy difference between these states.
The exchange interaction is related to the Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated ...
, which says that two electrons with the same spin cannot also be in the same spatial state (orbital). This is a consequence of the spin–statistics theorem
In quantum mechanics, the spin–statistics theorem relates the intrinsic spin of a particle (angular momentum not due to the orbital motion) to the particle statistics it obeys. In units of the reduced Planck constant ''ħ'', all particles tha ...
and that electrons are fermions
In particle physics, a fermion is a particle that follows Fermi–Dirac statistics. Generally, it has a half-odd-integer spin: spin , spin , etc. In addition, these particles obey the Pauli exclusion principle. Fermions include all quarks and ...
. Therefore, under certain conditions, when the orbitals of the unpaired outer valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
s from adjacent atoms overlap, the distributions of their electric charge in space are farther apart when the electrons have parallel spins than when they have opposite spins. This reduces the electrostatic energy of the electrons when their spins are parallel compared to their energy when the spins are antiparallel, so the parallel-spin state is more stable. This difference in energy is called the exchange energy
In chemistry and physics, the exchange interaction (with an exchange energy and exchange term) is a quantum mechanical effect that only occurs between identical particles. Despite sometimes being called an exchange force in an analogy to classica ...
. In simple terms, the outer electrons of adjacent atoms, which repel each other, can move further apart by aligning their spins in parallel, so the spins of these electrons tend to line up.
This energy difference can be orders of magnitude larger than the energy differences associated with the magnetic dipole–dipole interaction
Magnetic dipole–dipole interaction, also called dipolar coupling, refers to the direct interaction between two magnetic dipoles.
Suppose and are two magnetic dipole moments that are far enough apart that they can be treated as point dipoles i ...
due to dipole orientation, which tends to align the dipoles antiparallel. In certain doped semiconductor oxides RKKY interactions have been shown to bring about periodic longer-range magnetic interactions, a phenomenon of significance in the study of spintronic materials.
The materials in which the exchange interaction is much stronger than the competing dipole–dipole interaction are frequently called ''magnetic materials''. For instance, in iron (Fe) the exchange force is about 1000 times stronger than the dipole interaction. Therefore, below the Curie temperature virtually all of the dipoles in a ferromagnetic material will be aligned. In addition to ferromagnetism, the exchange interaction is also responsible for the other types of spontaneous ordering of atomic magnetic moments occurring in magnetic solids, antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
and ferrimagnetism
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
.
There are different exchange interaction mechanisms which create the magnetism in different ferromagnetic, ferrimagnetic, and antiferromagnetic substances. These mechanisms include direct exchange, RKKY exchange, double exchange The double-exchange mechanism is a type of a magnetic exchange that may arise between ions in different oxidation states. First proposed by Clarence Zener, this theory predicts the relative ease with which an electron may be exchanged between two s ...
, and superexchange.
Magnetic anisotropy
Although the exchange interaction keeps spins aligned, it does not align them in a particular direction. Withoutmagnetic anisotropy
In condensed matter physics, magnetic anisotropy describes how an object's magnetic properties can be different depending on direction. In the simplest case, there is no preferential direction for an object's magnetic moment. It will respond ...
, the spins in a magnet randomly change direction in response to thermal fluctuations
In statistical mechanics, thermal fluctuations are random deviations of a system from its average state, that occur in a system at equilibrium.In statistical mechanics they are often simply referred to as fluctuations. All thermal fluctuations b ...
and the magnet is superparamagnetic. There are several kinds of magnetic anisotropy, the most common of which is magnetocrystalline anisotropy
In physics, a ferromagnetic material is said to have magnetocrystalline anisotropy if it takes more energy to magnetize it in certain directions than in others. These directions are usually related to the principal axes of its crystal lattice. I ...
. This is a dependence of the energy on the direction of magnetization relative to the crystallographic lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
. Another common source of anisotropy, inverse magnetostriction, is induced by internal strains. Single-domain magnets also can have a ''shape anisotropy'' due to the magnetostatic effects of the particle shape. As the temperature of a magnet increases, the anisotropy tends to decrease, and there is often a blocking temperature at which a transition to superparamagnetism occurs.
Magnetic domains
 The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called ''
The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called ''magnetic domain
A magnetic domain is a region within a magnetic material in which the magnetization is in a uniform direction. This means that the individual magnetic moments of the atoms are aligned with one another and they point in the same direction. When c ...
s''
(also known as ''Weiss domains''). Within each domain, the spins are aligned, but (if the bulk material is in its lowest energy configuration; i.e. ''unmagnetized''), the spins of separate domains point in different directions and their magnetic fields cancel out, so the object has no net large scale magnetic field.
Ferromagnetic materials spontaneously divide into magnetic domains because the ''exchange interaction
In chemistry and physics, the exchange interaction (with an exchange energy and exchange term) is a quantum mechanical effect that only occurs between identical particles. Despite sometimes being called an exchange force in an analogy to classical ...
'' is a short-range force, so over long distances of many atoms the tendency of the magnetic dipoles to reduce their energy by orienting in opposite directions wins out. If all the dipoles in a piece of ferromagnetic material are aligned parallel, it creates a large magnetic field extending into the space around it. This contains a lot of magnetostatic
Magnetostatics is the study of magnetic fields in systems where the currents are steady (not changing with time). It is the magnetic analogue of electrostatics, where the charges are stationary. The magnetization need not be static; the equati ...
energy. The material can reduce this energy by splitting into many domains pointing in different directions, so the magnetic field is confined to small local fields in the material, reducing the volume of the field. The domains are separated by thin domain walls
A domain wall is a type of topological soliton that occurs whenever a discrete symmetry is spontaneously broken. Domain walls are also sometimes called kinks in analogy with closely related kink solution of the sine-Gordon model or models with pol ...
a number of molecules thick, in which the direction of magnetization of the dipoles rotates smoothly from one domain's direction to the other.
Magnetized materials
 Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the
Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the Barkhausen effect
The Barkhausen effect is a name given to the noise in the magnetic output of a ferromagnet when the magnetizing force applied to it is changed. Discovered by German physicist Heinrich Barkhausen in 1919, it is caused by rapid changes of size o ...
: as the magnetizing field is changed, the magnetization changes in thousands of tiny discontinuous jumps as the domain walls suddenly "snap" past defects.
This magnetization as a function of the external field is described by a hysteresis curve. Although this state of aligned domains found in a piece of magnetized ferromagnetic material is not a minimal-energy configuration, it is metastable
In chemistry and physics, metastability denotes an intermediate Energy level, energetic state within a dynamical system other than the system's ground state, state of least energy.
A ball resting in a hollow on a slope is a simple example of me ...
, and can persist for long periods, as shown by samples of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the ...
from the sea floor which have maintained their magnetization for millions of years.
Heating and then cooling ( annealing) a magnetized material, subjecting it to vibration by hammering it, or applying a rapidly oscillating magnetic field from a degaussing coil tends to release the domain walls from their pinned state, and the domain boundaries tend to move back to a lower energy configuration with less external magnetic field, thus ''demagnetizing
Degaussing is the process of decreasing or eliminating a remnant magnetic field. It is named after the gauss, a unit of magnetism, which in turn was named after Carl Friedrich Gauss. Due to magnetic hysteresis, it is generally not possible to re ...
'' the material.
Commercial magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, ...
s are made of "hard" ferromagnetic or ferrimagnetic materials with very large magnetic anisotropy
In condensed matter physics, magnetic anisotropy describes how an object's magnetic properties can be different depending on direction. In the simplest case, there is no preferential direction for an object's magnetic moment. It will respond ...
such as alnico
Alnico is a family of iron alloys which in addition to iron are composed primarily of aluminium (Al), nickel (Ni), and cobalt (Co), hence the acronym ''al-ni-co''. They also include copper, and sometimes titanium. Alnico alloys are ferromagnetic, ...
and ferrites, which have a very strong tendency for the magnetization to be pointed along one axis of the crystal, the "easy axis". During manufacture the materials are subjected to various metallurgical processes in a powerful magnetic field, which aligns the crystal grains so their "easy" axes of magnetization all point in the same direction. Thus the magnetization, and the resulting magnetic field, is "built in" to the crystal structure of the material, making it very difficult to demagnetize.
Curie temperature
As the temperature increases, thermal motion, orentropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
, competes with the ferromagnetic tendency for dipoles to align. When the temperature rises beyond a certain point, called the Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
, there is a second-order phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of ...
and the system can no longer maintain a spontaneous magnetization, so its ability to be magnetized or attracted to a magnet disappears, although it still responds paramagnetically to an external field. Below that temperature, there is a spontaneous symmetry breaking
Spontaneous symmetry breaking is a spontaneous process of symmetry breaking, by which a physical system in a symmetric state spontaneously ends up in an asymmetric state. In particular, it can describe systems where the equations of motion or the ...
and magnetic moments become aligned with their neighbors. The Curie temperature itself is a critical point, where the magnetic susceptibility
In electromagnetism, the magnetic susceptibility (Latin: , "receptive"; denoted ) is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization (magnetic moment per unit volume) to the ap ...
is theoretically infinite and, although there is no net magnetization, domain-like spin correlations fluctuate at all length scales.
The study of ferromagnetic phase transitions, especially via the simplified Ising Ising is a surname. Notable people with the surname include:
* Ernst Ising (1900–1998), German physicist
* Gustav Ising (1883–1960), Swedish accelerator physicist
* Rudolf Ising, animator for ''MGM'', together with Hugh Harman often credited ...
spin model, had an important impact on the development of statistical physics. There, it was first clearly shown that mean field theory
In physics and probability theory, Mean-field theory (MFT) or Self-consistent field theory studies the behavior of high-dimensional random (stochastic) models by studying a simpler model that approximates the original by averaging over degrees of ...
approaches failed to predict the correct behavior at the critical point (which was found to fall under a ''universality class'' that includes many other systems, such as liquid-gas transitions), and had to be replaced by renormalization group
In theoretical physics, the term renormalization group (RG) refers to a formal apparatus that allows systematic investigation of the changes of a physical system as viewed at different scales. In particle physics, it reflects the changes in the ...
theory.
See also
* * * * * *References
External links
*Electromagnetism
– ch. 11, from an online textbook * Detailed nonmathematical description of ferromagnetic materials with illustrations
Magnetism: Models and Mechanisms
in E. Pavarini, E. Koch, and U. Schollwöck: Emergent Phenomena in Correlated Matter, Jülich 2013, {{Authority control Quantum phases Magnetic hysteresis Physical phenomena Ferromagnetism