Ei Mechanism on:
[Wikipedia]
[Google]
[Amazon]
In
 The elimination must be ''syn'' and the atoms coplanar for four and five-membered transition states,Branko, J. (1997). "Theoretical studies of thermal syn elimination reaction of organic amine oxide, sulfoxide and phosphoxide by ab initio and density functional methods". ''Theo. Chem.'' 389 257-263. but
The elimination must be ''syn'' and the atoms coplanar for four and five-membered transition states,Branko, J. (1997). "Theoretical studies of thermal syn elimination reaction of organic amine oxide, sulfoxide and phosphoxide by ab initio and density functional methods". ''Theo. Chem.'' 389 257-263. but  There is a substantial amount of evidence to support the existence of the Ei mechanism such as: 1) the
There is a substantial amount of evidence to support the existence of the Ei mechanism such as: 1) the  The pyrolysis of ''N,N''-dimethyl-2-phenylcyclohexylamine-N-oxide shows how conformational effects and the stability of the transition state affect product composition for cyclic substrates.
The pyrolysis of ''N,N''-dimethyl-2-phenylcyclohexylamine-N-oxide shows how conformational effects and the stability of the transition state affect product composition for cyclic substrates.
 In the ''
In the ''


 Allylic alcohols can be formed from β-hydroxy phenyl sulfoxides that contain a β’-hydrogen through an Ei mechanism, tending to give the β,γ- unsaturation.OKawara, R.; Ueta, K.; Nokami, J. (1978). "Pyrolysis of B-Hydroxy Sulfoxides II. Synthesis of Allylic alcohols". ''Tetrahedron Lett.'' 49 4903-4904.
Allylic alcohols can be formed from β-hydroxy phenyl sulfoxides that contain a β’-hydrogen through an Ei mechanism, tending to give the β,γ- unsaturation.OKawara, R.; Ueta, K.; Nokami, J. (1978). "Pyrolysis of B-Hydroxy Sulfoxides II. Synthesis of Allylic alcohols". ''Tetrahedron Lett.'' 49 4903-4904.
 1,3-Dienes were found to be formed upon the treatment of an allylic alcohol with an aryl sulfide in the presence of
1,3-Dienes were found to be formed upon the treatment of an allylic alcohol with an aryl sulfide in the presence of 
 The Chugaev elimination is very similar to the ester pyrolysis, but requires significantly lower temperatures to achieve the elimination, thus making it valuable for rearrangement-prone substrates.
The Chugaev elimination is very similar to the ester pyrolysis, but requires significantly lower temperatures to achieve the elimination, thus making it valuable for rearrangement-prone substrates.
 First, the alcohol displaces the triethylamine on the
First, the alcohol displaces the triethylamine on the

 The mechanism for this reaction is analogous to the sulfoxide elimination, which is a thermal ''syn'' elimination through a 5-membered cyclic transition state. Selenoxides are preferred for this type of transformation over sulfoxides due to their increased reactivity toward β-elimination, in some cases allowing the elimination to take place at room temperature.
The mechanism for this reaction is analogous to the sulfoxide elimination, which is a thermal ''syn'' elimination through a 5-membered cyclic transition state. Selenoxides are preferred for this type of transformation over sulfoxides due to their increased reactivity toward β-elimination, in some cases allowing the elimination to take place at room temperature.
 The areneselenic acid generated after the elimination step is in equilibrium with the
The areneselenic acid generated after the elimination step is in equilibrium with the
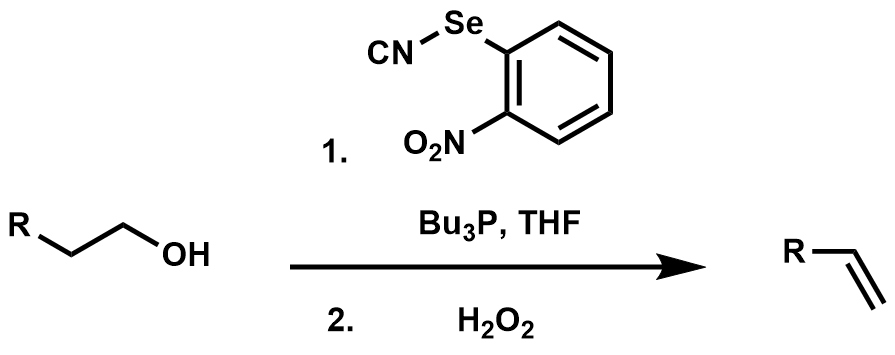 The reaction begins with the formation of a selenophosphonium salt which reacts with the alcohol to form an oxaphosphonium salt. The aryl selenium anion displaces tributylphosphine oxide forming the alkyl aryl selenide species. The selenide is then treated with excess hydrogen peroxide leading to the selenoxide which eliminates the β-hydrogen through a 5-member cyclic transition state, yielding an alkene.
The reaction begins with the formation of a selenophosphonium salt which reacts with the alcohol to form an oxaphosphonium salt. The aryl selenium anion displaces tributylphosphine oxide forming the alkyl aryl selenide species. The selenide is then treated with excess hydrogen peroxide leading to the selenoxide which eliminates the β-hydrogen through a 5-member cyclic transition state, yielding an alkene.
 The
The
 Cyclic amine oxides (5, 7-10-membered nitrogen containing rings) can also undergo internal ''syn'' elimination to yield acyclic hydroxylamines containing terminal alkenes.
Cyclic amine oxides (5, 7-10-membered nitrogen containing rings) can also undergo internal ''syn'' elimination to yield acyclic hydroxylamines containing terminal alkenes.
 The Wittig modified Hofmann elimination goes through the same Ei mechanism, but instead of using
The Wittig modified Hofmann elimination goes through the same Ei mechanism, but instead of using
 The scope of this reaction does not include primary alkyl iodides because the iodoso intermediate rearranges to the hypoiodite intermediate, which, under the reaction conditions, is converted to an alcohol. Strongly electron-withdrawing groups suppress the rearrangement pathway, allowing the pericyclic ''syn'' elimination pathway to predominate.
The scope of this reaction does not include primary alkyl iodides because the iodoso intermediate rearranges to the hypoiodite intermediate, which, under the reaction conditions, is converted to an alcohol. Strongly electron-withdrawing groups suppress the rearrangement pathway, allowing the pericyclic ''syn'' elimination pathway to predominate.
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, the Ei mechanism (Elimination Internal/Intramolecular), also known as a thermal syn elimination or a pericyclic syn elimination, is a special type of elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
in which two vicinal (adjacent) substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
s on an alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which ...
framework leave simultaneously via a cyclic transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
to form an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
in a ''syn'' elimination. This type of elimination is unique because it is thermally activated and does not require additional reagents, unlike regular eliminations, which require an acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
or base, or would in many cases involve charged intermediates. This reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
is often found in pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
.
General features
Compounds that undergo elimination through cyclic transition states upon heating, with no other reagents present, are given the designation as Ei reactions. Depending on the compound, elimination takes place through a four, five, or six-membered transition state.Carey, F. A.; Sunburg, R. J. ''Advanced Organic Chemistry: Reaction and Synthesis'', 5th Ed.; Part B; Springer: New York, 2010 The elimination must be ''syn'' and the atoms coplanar for four and five-membered transition states,Branko, J. (1997). "Theoretical studies of thermal syn elimination reaction of organic amine oxide, sulfoxide and phosphoxide by ab initio and density functional methods". ''Theo. Chem.'' 389 257-263. but
The elimination must be ''syn'' and the atoms coplanar for four and five-membered transition states,Branko, J. (1997). "Theoretical studies of thermal syn elimination reaction of organic amine oxide, sulfoxide and phosphoxide by ab initio and density functional methods". ''Theo. Chem.'' 389 257-263. but coplanarity
In geometry, a set of points in space are coplanar if there exists a geometric plane that contains them all. For example, three points are always coplanar, and if the points are distinct and non-collinear, the plane they determine is unique. Howe ...
is not required for six-membered transition states.
 There is a substantial amount of evidence to support the existence of the Ei mechanism such as: 1) the
There is a substantial amount of evidence to support the existence of the Ei mechanism such as: 1) the kinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical ki ...
of the reactions were found to be first order,O’Connor, G.L.; Nace, H. R. (1953). "Further Studies on the Chugaev Reaction and Related Reactions". ''J. Am. Chem. Soc.'' 75 2118-. 2) the use of free-radical
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
inhibitors did not affect the rate of the reactions, indicating no free-radical mechanisms are involved Barton, D.H.R.; Head, A.J.; Williams, R.J. (1953). "Stereospecificity in Thermal Elimination Reactions. Part III. The Pyrolysis of (−)-Menthyl Benzoate". ''J. Chem Soc. 453 1715- .Anslyn, E. V.; Dougherty, D. A. ''Modern Physical Organic Chemistry'', Murdzek, J., Ed. University Science Books, 2006. 3) isotope studies for the Cope elimination
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of the N-oxide to form an alkene and a hydroxylamine.
Mechanism and applications
The reaction mechanism involves an intramolecular 5-membered cyclic tr ...
indicate the C-H and C-N bonds are partially broken in the transition state,Wright, D.R.; Sims, L.B.; Fry, A. (1983). "Carbon-14 kinetic isotope effects and kinetic studies in the syn-elimination reactions of (2-phenylethyl)dimethylamine oxides". ''J. Am. Chem. Soc.'' 105 3714-. this is also supported by computations
Computation is any type of arithmetic or non-arithmetic calculation that follows a well-defined model (e.g., an algorithm).
Mechanical or electronic devices (or, historically, people) that perform computations are known as ''computers''. An espe ...
that show bond lengthening in the transition state Kahn, S.D; Erickson, J.A. (1994). "Theoretical Studies of Thermal Syn Elimination Reactions. The Relative Rates of Ethyl Formate, Ethyl Xanthate, and Ethyl Phosphinate Eliminations". ''J. Am. Chem. Soc.'' 116 6271-6276. and 4) without the intervention of other mechanisms, the Ei mechanism gives exclusively syn elimination products.
There are many factors that affect the product composition of Ei reactions, but typically they follow Hofmann’s rule and lose a β-hydrogen from the least substituted position, giving the alkene that is less substituted (the opposite of Zaitsev's rule
In organic chemistry, Zaitsev's rule (or Saytzeff's rule, Saytzev's rule) is an empirical rule for predicting the favored alkene product(s) in elimination reactions. While at the University of Kazan, Russian chemist Alexander Zaitsev studied a vari ...
). Some factors affecting product composition include steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
, conjugation
Conjugation or conjugate may refer to:
Linguistics
* Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
* Complex conjugation, the chang ...
, and stability
Stability may refer to:
Mathematics
*Stability theory, the study of the stability of solutions to differential equations and dynamical systems
**Asymptotic stability
**Linear stability
**Lyapunov stability
**Orbital stability
**Structural stabilit ...
of the forming alkene.
For acyclic substrates, the ''Z''-isomer is typically the minor product due to the destabilizing gauche interaction in the transition state, but the selectivity is not usually high.
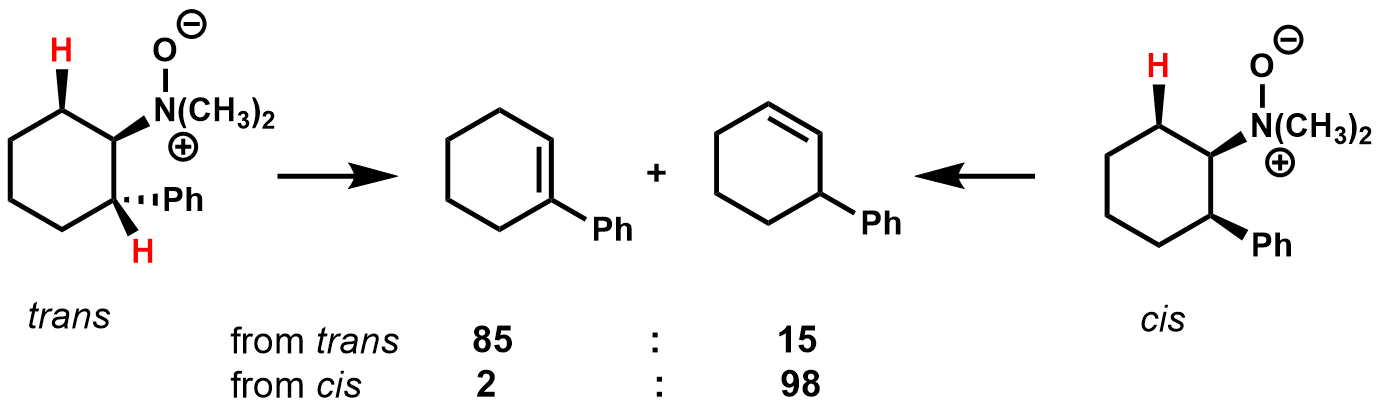
 The pyrolysis of ''N,N''-dimethyl-2-phenylcyclohexylamine-N-oxide shows how conformational effects and the stability of the transition state affect product composition for cyclic substrates.
The pyrolysis of ''N,N''-dimethyl-2-phenylcyclohexylamine-N-oxide shows how conformational effects and the stability of the transition state affect product composition for cyclic substrates.
 In the ''
In the ''trans
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of".
Used alone, trans may refer to:
Arts, entertainment, and media
* Trans (festival), a former festival in Belfast, Northern Ireland, United Kingdom
* ''Trans'' (film ...
'' isomer, there are two ''cis''-β-hydrogens that can eliminate. The major product is the alkene that is in conjugation with the phenyl ring, presumably due to the stabilizing effect on the transition state. In the ''cis
Cis or cis- may refer to:
Places
* Cis, Trentino, in Italy
* In Poland:
** Cis, Świętokrzyskie Voivodeship, south-central
** Cis, Warmian-Masurian Voivodeship, north
Math, science and biology
* cis (mathematics) (cis(''θ'')), a trigonome ...
'' isomer, there is only one ''cis''-''B''-hydrogen that can eliminate, giving the nonconjugated regioisomer as the major product.
Ester (acetate) pyrolysis
The pyrolytic decomposition ofester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s is an example of a thermal ''syn'' elimination. When subjected to temperatures above 400 °C, esters containing β-hydrogens can eliminate a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
through a 6-membered transition state, resulting in an alkene.

Isotopic labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific ...
was used to confirm that ''syn'' elimination occurs during ester pyrolysis in the formation of stilbene Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene:
* (''E'')-Stilbene (''trans'' isomer)
* (''Z'')-Stilbene (''cis'' isomer)
See also
* Stilbenoids, a class of molecules found in plants
* 1,1-Diphenylethylene
1,1-Diphenyl ...
.Curtin, D.Y.; Kellom, D.B. (1953). "Elimination and Replacement Reactions of dl-erythro- and dl-threo-2-Deutero-1,2-diphenylethanol and Derivatives". ''J. Am. Chem. Soc.'' 75 6011-.

Sulfur-based
Sulfoxide elimination
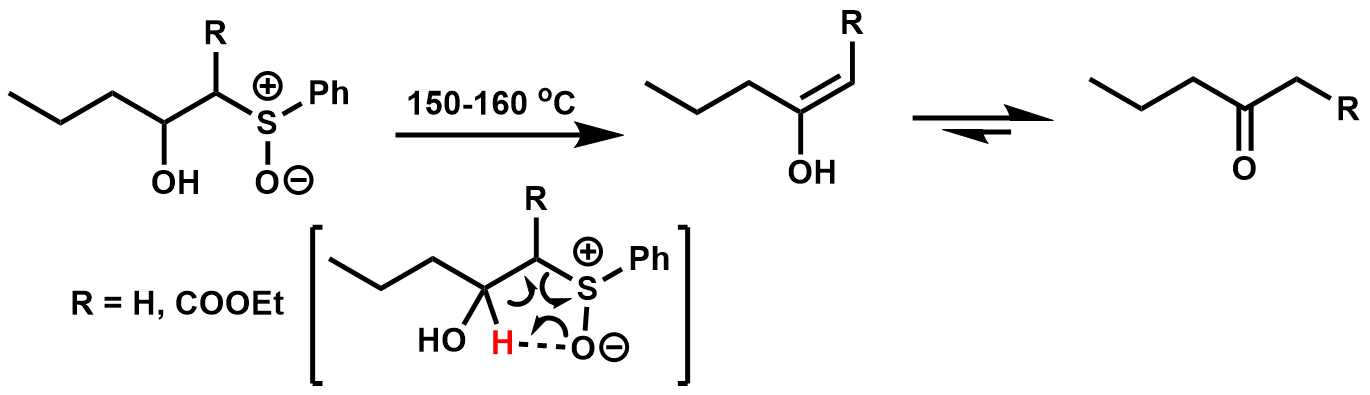
β-hydroxy phenyl sulfoxides were found to undergo thermal elimination through a 5-membered cyclic transition state, yielding β-keto esters and methyl ketones aftertautomerization
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
and a sulfenic acid
In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula . It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids () and sulfonic acids (), respectively. The base ...
.Kinoshita, M.; Kunieda, N.; Nokami, J. (1975). "Pyrolysis of B-Hydroxy Sulfoxides to Ketones". ''Tetrahedron Lett.'' 33 2841-2844.
 Allylic alcohols can be formed from β-hydroxy phenyl sulfoxides that contain a β’-hydrogen through an Ei mechanism, tending to give the β,γ- unsaturation.OKawara, R.; Ueta, K.; Nokami, J. (1978). "Pyrolysis of B-Hydroxy Sulfoxides II. Synthesis of Allylic alcohols". ''Tetrahedron Lett.'' 49 4903-4904.
Allylic alcohols can be formed from β-hydroxy phenyl sulfoxides that contain a β’-hydrogen through an Ei mechanism, tending to give the β,γ- unsaturation.OKawara, R.; Ueta, K.; Nokami, J. (1978). "Pyrolysis of B-Hydroxy Sulfoxides II. Synthesis of Allylic alcohols". ''Tetrahedron Lett.'' 49 4903-4904.
 1,3-Dienes were found to be formed upon the treatment of an allylic alcohol with an aryl sulfide in the presence of
1,3-Dienes were found to be formed upon the treatment of an allylic alcohol with an aryl sulfide in the presence of triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
.Wollowitz, S.; Reich, H.J. (1982). "Conversion of Allyl Alcohols to 1,3-Dienes by Sequential Sulfenate-Sulfoxide ,3Sigmatropic Rearrangement and Syn Elimination". ''J. Am. Chem. Soc.'' 104 7051-7059. Initially, a sulfenate ester is formed followed by a ,3sigmatropic rearrangement to afford an allylic sulfoxide which undergoes thermal ''syn'' elimination to yield the 1,3-diene.

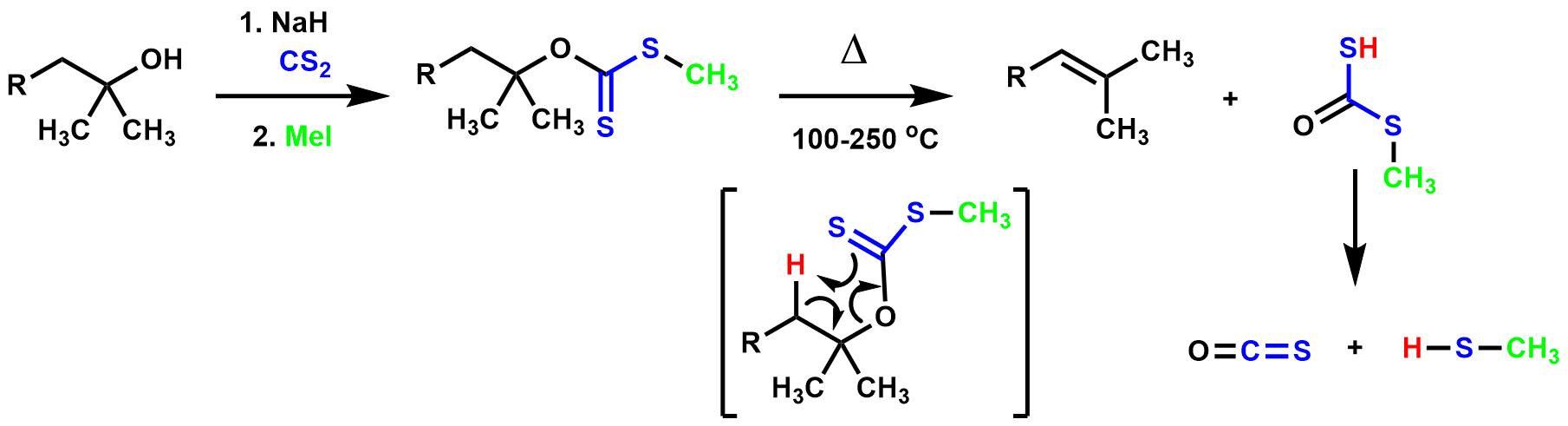
Chugaev elimination
TheChugaev elimination
The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev (1873-1922), who ...
is the pyrolysis of a xanthate ester, resulting in an olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
.Kurti, L.; Czako, B. ''Strategic Applications of Named Reactions in Organic Synthesis'', Academic Press, 2005. To form the xanthate ester, an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
reacts with carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non ...
in the presence of a base, resulting in a metal xanthate which is trapped with an alkylating agent
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
(typically methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
). The olefin is formed through the thermal ''syn'' elimination of the β-hydrogen and xanthate ester. The reaction is irreversible because the resulting by-products, carbonyl sulfide
Carbonyl sulfide is the chemical compound with the linear formula OCS. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom. Carbonyl sulfide can be conside ...
and methanethiol
Methanethiol (also known as methyl mercaptan) is an organosulfur compound with the chemical formula . It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals (including hum ...
, are very stable.
 The Chugaev elimination is very similar to the ester pyrolysis, but requires significantly lower temperatures to achieve the elimination, thus making it valuable for rearrangement-prone substrates.
The Chugaev elimination is very similar to the ester pyrolysis, but requires significantly lower temperatures to achieve the elimination, thus making it valuable for rearrangement-prone substrates.
Burgess dehydration reaction
The dehydration of secondary and tertiary alcohols to yield an olefin through a sulfamate ester intermediate is called the Burgess dehydration reaction.Taylor, E.A.; Penton, H.R.Jr.; Burgess, E.M. (1970). "Synthetic Applications of N-Carboalkoxysulfamate Esters". ''J. Am. Chem. Soc.'' 92 5224-5226.Taylor, E.A.; Penton, H.R.Jr.; Burgess, E.M. (1973). "Thermal Reactions of Alkyl N-Carbomethoxysulfamate Esters". ''J. Org. Chem.'' 38 26-. The reaction conditions used are typically very mild, giving it some advantage over other dehydration methods for sensitive substrates. This reaction was used during the first total synthesis oftaxol
Paclitaxel (PTX), sold under the brand name Taxol among others, is a chemotherapy medication used to treat a number of types of cancer. This includes ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cance ...
to install an exo-methylene group on the C ring.Holton, R.A.; et al. (1994). "First Total Synthesis of Taxol. 2. Completion of the C and D Rings". ''J. Am. Chem. Soc.'' 116 1599-1600.
 First, the alcohol displaces the triethylamine on the
First, the alcohol displaces the triethylamine on the Burgess reagent
The Burgess reagent (methyl ''N''-(triethylammoniumsulfonyl)carbamate) is a mild and selective dehydrating reagent often used in organic chemistry. It was developed in the laboratory of Edward M. Burgess at Georgia Tech.
The Burgess reagent is ...
, forming the sulfamate ester intermediate. β-hydrogen abstraction and elimination of the sulfamate ester through a 6-membered cyclic transition state yields the alkene.
Thiosulfinate elimination
Thiosulfinate
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R (R are organic substituents). Thiolsulfinates are also named as alkanethiosulfinic (or arenethiosulfinic) acid esters. They are the first member of ...
s can eliminate in the manner analogous to sulfoxides. Representative is the fragmentation of allicin
Allicin is an organosulfur compound obtained from garlic, a species in the family Alliaceae. It was first isolated and studied in the laboratory by Chester J. Cavallito and John Hays Bailey in 1944. When fresh garlic is chopped or crushed, the ...
to thioacrolein, which will go on to form vinyldithiin
Vinyldithiins, more precisely named 3-vinyl-4''H''-1,2-dithiin and 2-vinyl-4''H''-1,3-dithiin, are organosulfur phytochemicals formed in the breakdown of allicin from crushed garlic (''Allium sativum''). Vinyldithiins are Diels-Alder dimers of thi ...
s. Such reactions are important in the antioxidant chemistry of garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plant in the genus ''Allium''. Its close relatives include the onion, shallot, leek, chive, Allium fistulosum, Welsh onion and Allium chinense, Chinese onion. It is native to South A ...
and other plants of the genus Allium
''Allium'' is a genus of monocotyledonous flowering plants that includes hundreds of species, including the cultivated onion, garlic, scallion, shallot, leek, and chives. The generic name ''Allium'' is the Latin word for garlic,Gledhill, Davi ...
.

Selenium-based
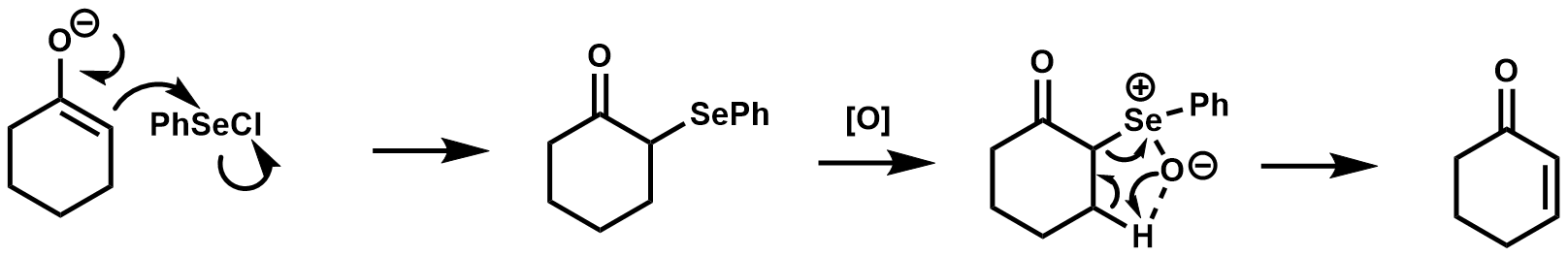
Selenoxide elimination
Theselenoxide elimination
Selenoxide elimination (also called α-selenation) is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is me ...
has been used in converting ketones, esters, and aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s to their α,β-unsaturated derivatives.Lauer, R.F; Young, M.W.; Sharpless, K.B. (1973). "Reactions of Selenoxides: Thermal Syn elimination and H2O-18 Exchange". ''Tetrahedron Lett.'' 22 1979-1982.
 The mechanism for this reaction is analogous to the sulfoxide elimination, which is a thermal ''syn'' elimination through a 5-membered cyclic transition state. Selenoxides are preferred for this type of transformation over sulfoxides due to their increased reactivity toward β-elimination, in some cases allowing the elimination to take place at room temperature.
The mechanism for this reaction is analogous to the sulfoxide elimination, which is a thermal ''syn'' elimination through a 5-membered cyclic transition state. Selenoxides are preferred for this type of transformation over sulfoxides due to their increased reactivity toward β-elimination, in some cases allowing the elimination to take place at room temperature.
 The areneselenic acid generated after the elimination step is in equilibrium with the
The areneselenic acid generated after the elimination step is in equilibrium with the diphenyl diselenide
Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This orange-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis.
Preparation a ...
which can react with olefins to yield β-hydroxy selenides under acidic or neutral conditions. Under basic conditions, this side reaction is suppressed.Reich, H.J.; Wollowitz, S.; Trend, J.E.; Chow, F.; Wendelborn, D.F. (1978). "Syn Elimination of Alkyl Selenoxides. Side Reactions Involving Selenenic Acids. Structural and Solvent Effects on Rates". ''J. Org. Chem.'' 43 1697-.
Grieco elimination
The one-pot dehydration of a primary alcohol to give an alkene through an ''o''-nitrophenyl selenoxide intermediate is called theGrieco elimination
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene. It is named for Paul Grieco.
The alcohol first reacts with ''o''-nitrophenylselenocyanate an ...
.Grieco, P.A.; Gilman, S.; Nishizawa, M. (1976). "Organoselenium Chemistry. A Facile One-Step Synthesis of Alkyl Aryl Selenides from Alcohols". ''J. Org. Chem.'' 41 1485-.Young, M.W.; Sharpless, B.K. (1975). "Olefin Synthesis. Rate Enhancement of the Elimination of Alkyl Aryl Selenoxides by Electron-Withdrawing Substituents". ''J. Org. Chem.'' 40 947-.
 The reaction begins with the formation of a selenophosphonium salt which reacts with the alcohol to form an oxaphosphonium salt. The aryl selenium anion displaces tributylphosphine oxide forming the alkyl aryl selenide species. The selenide is then treated with excess hydrogen peroxide leading to the selenoxide which eliminates the β-hydrogen through a 5-member cyclic transition state, yielding an alkene.
The reaction begins with the formation of a selenophosphonium salt which reacts with the alcohol to form an oxaphosphonium salt. The aryl selenium anion displaces tributylphosphine oxide forming the alkyl aryl selenide species. The selenide is then treated with excess hydrogen peroxide leading to the selenoxide which eliminates the β-hydrogen through a 5-member cyclic transition state, yielding an alkene.
 The
The electron-withdrawing
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
nitro group
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitr ...
was found to increase both the rate of elimination and the final yield of the olefin.
Nitrogen-based
Cope elimination
TheCope elimination
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of the N-oxide to form an alkene and a hydroxylamine.
Mechanism and applications
The reaction mechanism involves an intramolecular 5-membered cyclic tr ...
(Cope reaction) is the elimination of a tertiary amine oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that contains the functional group , a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-grou ...
to yield an alkene and a hydroxylamine
Hydroxylamine is an inorganic compound with the formula . The material is a white crystalline, hygroscopic compound.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–43 ...
through an Ei mechanism.Cope, A.C.; Foster, T.T.; Towle, P.H. (1949). "Thermal Decomposition of Amine Oxides to Olefins and Dialkylhydroxylamines". ''J. Am. Chem. Soc.'' 71 3929-. The Cope elimination was used in the synthesis of a mannopyranosylamine mimic.Vasella, A.; Remen, L. (2002). "Conformationally Biased Mimics of Mannopyranosylamines: Inhibitors of B-Mannosidases?". ''Helv. Chim. Acta.'' 85 1118-. The tertiary amine was oxidized to the amine oxide using ''m''-chloroperoxybenzoic acid (''m''CPBA) and subjected to high temperatures for thermal ''syn'' elimination of the β-hydrogen and amine oxide through a cyclic transition state, yielding the alkene. It is worth noting that the indicated hydrogen (in green) is the only hydrogen available for ''syn'' elimination.
 Cyclic amine oxides (5, 7-10-membered nitrogen containing rings) can also undergo internal ''syn'' elimination to yield acyclic hydroxylamines containing terminal alkenes.
Cyclic amine oxides (5, 7-10-membered nitrogen containing rings) can also undergo internal ''syn'' elimination to yield acyclic hydroxylamines containing terminal alkenes.
Special cases for the Hofmann elimination
The mechanism for theHofmann elimination
Hofmann elimination is an elimination reaction of an amine to form alkenes. The least stable alkene (the one with the least number of substituents on the carbons of the double bond), called the Hofmann product, is formed. This tendency, known as ...
is generally E2, but can go through an Ei pathway under certain circumstances. For some sterically hindered molecules the base deprotonates a methyl group on the amine instead of the β-hydrogen directly, forming an ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms h ...
intermediate which eliminates trimethylamine through a 5-membered transition state, forming the alkene. Deuterium labeling studies confirmed this mechanism by observing the formation of deuterated trimethylamine (and no deuterated water, which would form from the E2 mechanism).Cope, A.C.; Mehta, A.S. (1963). "Mechanism of the Hofmann Elimination Reaction: An Ylide Intermediate in the Pyrolysis of a Highly Branched Quaternary Hydroxide". ''J. Am. Chem. Soc.'' 85 1949-.
 The Wittig modified Hofmann elimination goes through the same Ei mechanism, but instead of using
The Wittig modified Hofmann elimination goes through the same Ei mechanism, but instead of using silver oxide
Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.
Preparation
Silver oxide can be prepared by combining aqueous solutions of silver nitrate and a ...
and water as base, the Wittig modification uses strong bases like alkylithiums or KNH2/liquid NH3.Wittig, G.; Polster, R. (1957). ''Ann. Chem.'' 102 612-.Bach, R.D.; Bair, K.W.; Andrzejewski, D. (1972). "The Wittig Modification of the Hofmann Elimination Reaction. Evidence for an alpha’, beta Mechanism". ''J. Am. Chem. Soc.'' 94 8608-.
Iodoso elimination
Secondary and tertiary alkyl iodides with strongly electron-withdrawing groups at theα-carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of ...
were found to undergo a pericyclic ''syn'' elimination when exposed to ''m''-chloroperbenzoic acid (''m''CPBA).Reich, H. J., Peake, S. L. (1978). “Hypervalent Organoiodine Chemistry. Syn Elimination of Alkyl Iodoso Compounds”. ''J. Am. Chem. Soc.'' 100 4888-. It is proposed that the reaction goes through an iodoso intermediate before the ''syn'' elimination of hypoiodous acid
Hypoiodous acid is the inorganic compound with the chemical formula HIO. It forms when an aqueous solution of iodine is treated with mercuric or silver salts. It rapidly decomposes by disproportionation:
: 5 HIO → HIO3 + 2 I2 ...
.
 The scope of this reaction does not include primary alkyl iodides because the iodoso intermediate rearranges to the hypoiodite intermediate, which, under the reaction conditions, is converted to an alcohol. Strongly electron-withdrawing groups suppress the rearrangement pathway, allowing the pericyclic ''syn'' elimination pathway to predominate.
The scope of this reaction does not include primary alkyl iodides because the iodoso intermediate rearranges to the hypoiodite intermediate, which, under the reaction conditions, is converted to an alcohol. Strongly electron-withdrawing groups suppress the rearrangement pathway, allowing the pericyclic ''syn'' elimination pathway to predominate.
References
{{Reaction mechanisms Elimination reactions