|
Meta-Chloroperoxybenzoic Acid
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. Preparation and purification mCPBA can be prepared by reacting m-Chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification. It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of ''m''-chlorobenzoic acid (10%) and water. The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5. Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so one can extract the acid impurity by careful control of pH. The purified material is reasonably stable against de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxyacetic Acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive. Peracetic acid is a weaker acid than the parent acetic acid, with a p''K''a of 8.2. Production Peracetic acid is produced industrially by the autoxidation of acetaldehyde: :O2 + CH3CHO → CH3CO3H It forms upon treatment of acetic acid with hydrogen peroxide with a strong acid catalyst: :H2O2 + CH3CO2H CH3CO3H + H2O As an alternative, acetyl chloride and acetic anhydride can be used to generate a solution of the acid with lower water content. Peracetic acid is generated ''in situ'' by some laundry detergents. This is achieved by the action of bleach activators, such as tetraacetylethylenediamine and sodium nonanoyloxybenzenesulfonate, upon hydrogen peroxide formed from sodium percarbonate in water. The peracetic acid is a more effective bleachin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyloin
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups. Synthesis Classic organic reactions exist for the synthesis of acyloins. * The acyloin condensation is a reductive coupling of esters * The benzoin condensation is condensation reaction between aldehydes catalyzed by a nucleophile * Oxidation of carbonyls is possible with molecular oxygen but not selective * Better alternative is oxidation of corresponding silyl enol ethers with ''m''CPBA in the Rubottom oxidation * MoOPH oxidation of carbonyls is a system with molybdenum peroxide, pyridine and hexamethylphosphoramide. Enolate oxidation by sulfonyloxaziridines Enolates can be oxidized by sulfonyloxaziridines. The enolate reacts by nucleophilic displacement at the electron deficient oxygen of the oxaziridine ring. : This rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agents
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate. Combust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorobenzenes
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals. Uses Historical The major use of chlorobenzene is as an intermediate in the production of herbicides, dyestuffs, and rubber. Chlorobenzene is also used as a high-boiling solvent in industrial applications as well as in the laboratory. Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, bis(2-nitrophenyl)disulfide, and 2-nitroaniline by nucleophilic displacement of the chloride, with respectively sodium hydroxide, sodium methoxide, sodium disulfide, and ammonia. The conversions of the 4-nitro derivative are similar. Chlorobenzene once was used in the manufacture of pesticides, most notably DDT, by reaction with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
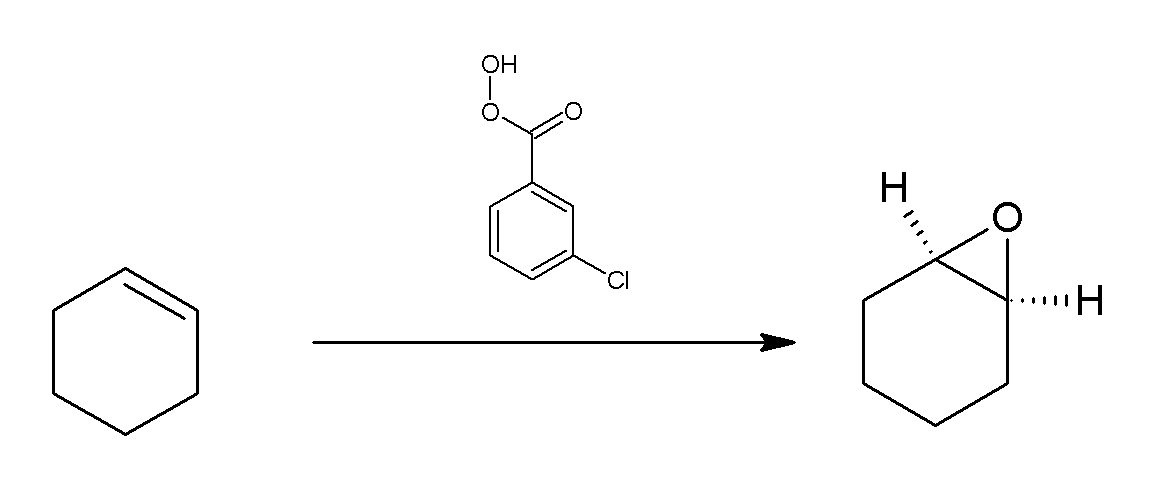
Reaction Of Cyclohexene With MCPBA
Reaction may refer to a process or to a response to an action, event, or exposure: Physics and chemistry *Chemical reaction *Nuclear reaction *Reaction (physics), as defined by Newton's third law *Chain reaction (other). Biology and medicine *Adverse drug reaction *Allergic reaction *Reflex, neural reaction *Hypersensitivity, immune reaction *Intolerance (other) * Light reaction (other). Psychology *Emotional, reaction *Reactivity (behaviour) *Proactivity, opposite of reactive behaviour *Reactive attachment disorder. Politics and culture *Reactionary, a political tendency *Reaction video *Commentary (other). Proper names and titles * ''Reaction'' (album), a 1986 album by American R&B singer Rebbie Jackson ** "Reaction" (song), the title song from the Rebbie Jackson album *"Reaction", a single by Dead Letter Circus *ReAction GUI, a GUI toolkit used on AmigaOS *Reaction.life, a political news and commentary website edited by Iain Martin *Re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides. Production and uses Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company. In the laboratory, it can be prepared by dehydration of cyclohexanol. : : Reactions and uses Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene. Cyclohexylbenzene is a precursor to both phenol and cyclohexanone. Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenated to give cyclohexanone, a precursor to caprolactam. The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxidant in the presence of a tungsten catalyst. Bromination gives 1,2-dibromocyclohexane. Structure Cyclohex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine Oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that contains the functional group , a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-group side chains attached to N. Sometimes it is written as →O or, incorrectly, as . In the strict sense, the term ''amine oxide'' applies only to oxides of tertiary amines. Sometimes it is also used for the analogous derivatives of primary and secondary amines. Examples of amine oxides include pyridine-''N''-oxide, a water-soluble crystalline solid with melting point 62–67 °C, and ''N''-methylmorpholine ''N''-oxide, which is an oxidant. Applications Amine oxides are surfactants commonly used in consumer products such as shampoos, conditioners, detergents, and hard surface cleaners. Alkyl dimethyl amine oxide (chain lengths C10–C16) is the most commercially used amine oxide. They are considered a high production volume class of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents. Synthesis and reactions By oxidation of thioethers and sulfoxides Sulfones are typically prepared by organic oxidation of thioethers, often referred to as sulfides. Sulfoxides are intermediates in this route. For example, dimethyl sulfide oxidizes to dimethyl sulfoxide and then to dimethyl sulfone. From SO2 : Sulfur dioxide is a convenient and widely used source of the sulfonyl functional group. Specifically, Sulfur dioxide participates in cycloaddition reactions with dienes. The industrially useful solvent sulfolane is prepared by addition of sulfur dioxide to buta-1,3-diene followed by hydrogenation of the resulting sulfolene. From sulfonyl and sulfuryl halides Sulfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent. Structure and bonding Sulfoxides feature relatively short S–O distances. In DMSO, the S–O distance is 1.531 Å. The sulfur center is pyramidal; the sum of the angles at sulfur is about 306°.. Sulfoxides are generally represented with the structural formula R−S(=O)−R', where R and R' are organic groups. The bond between the sulfur and oxygen atoms is intermediate of a dative bond and a polarized double bond. The double-bond resonance form implies 10 electrons around sulfur (10-S-3 in N-X-L notation). The double-bond character of the S−O bond may be accoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide. Chemical properties The sulfide ion, S2−, does not exist in aqueous alkaline solutions of Na2S. Instead sulfide converts to hydrosulfide: :S2− + H2O → SH− + OH− Upon treatment with an acid, sulfide salts convert to hydrogen sulfide: :S2− + H+ → SH− :SH− + H+ → H2S Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides, polythionates, sulfite, or sulfate. Metal sulfides react with halogens, forming sulfur and metal salts. :8 MgS + 8 I2 → S8 + 8 M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |