diffuse reflector on:
[Wikipedia]
[Google]
[Amazon]
 Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher
 There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the ''
There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the ''
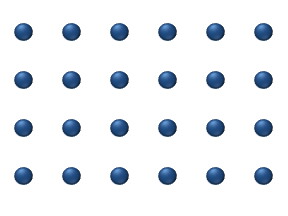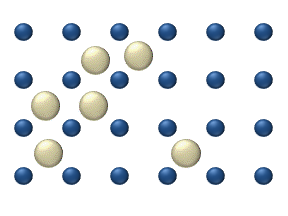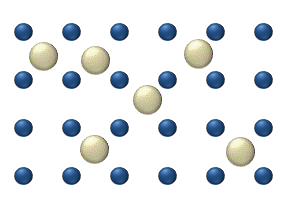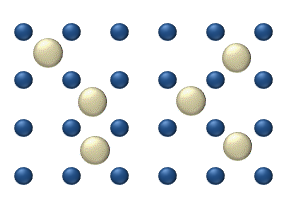 Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
:
Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
:
concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
or chemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process
In probability theory and related fields, a stochastic () or random process is a mathematical object usually defined as a family of random variables in a probability space, where the index of the family often has the interpretation of time. Sto ...
due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics
Statistics (from German language, German: ', "description of a State (polity), state, a country") is the discipline that concerns the collection, organization, analysis, interpretation, and presentation of data. In applying statistics to a s ...
, probability theory
Probability theory or probability calculus is the branch of mathematics concerned with probability. Although there are several different probability interpretations, probability theory treats the concept in a rigorous mathematical manner by expre ...
, information theory
Information theory is the mathematical study of the quantification (science), quantification, Data storage, storage, and telecommunications, communication of information. The field was established and formalized by Claude Shannon in the 1940s, ...
, neural networks
A neural network is a group of interconnected units called neurons that send signals to one another. Neurons can be either Cell (biology), biological cells or signal pathways. While individual neurons are simple, many of them together in a netwo ...
, finance
Finance refers to monetary resources and to the study and Academic discipline, discipline of money, currency, assets and Liability (financial accounting), liabilities. As a subject of study, is a field of Business administration, Business Admin ...
, and marketing
Marketing is the act of acquiring, satisfying and retaining customers. It is one of the primary components of Business administration, business management and commerce.
Marketing is usually conducted by the seller, typically a retailer or ma ...
.
The concept of diffusion is widely used in many fields, including physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
( particle diffusion), chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
, sociology
Sociology is the scientific study of human society that focuses on society, human social behavior, patterns of Interpersonal ties, social relationships, social interaction, and aspects of culture associated with everyday life. The term sociol ...
, economics
Economics () is a behavioral science that studies the Production (economics), production, distribution (economics), distribution, and Consumption (economics), consumption of goods and services.
Economics focuses on the behaviour and interac ...
, statistics
Statistics (from German language, German: ', "description of a State (polity), state, a country") is the discipline that concerns the collection, organization, analysis, interpretation, and presentation of data. In applying statistics to a s ...
, data science
Data science is an interdisciplinary academic field that uses statistics, scientific computing, scientific methods, processing, scientific visualization, algorithms and systems to extract or extrapolate knowledge from potentially noisy, stru ...
, and finance
Finance refers to monetary resources and to the study and Academic discipline, discipline of money, currency, assets and Liability (financial accounting), liabilities. As a subject of study, is a field of Business administration, Business Admin ...
(diffusion of people, ideas, data and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection.
A gradient
In vector calculus, the gradient of a scalar-valued differentiable function f of several variables is the vector field (or vector-valued function) \nabla f whose value at a point p gives the direction and the rate of fastest increase. The g ...
is the change in the value of a quantity; for example, concentration, pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
, or temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
with the change in another variable, usually distance
Distance is a numerical or occasionally qualitative measurement of how far apart objects, points, people, or ideas are. In physics or everyday usage, distance may refer to a physical length or an estimation based on other criteria (e.g. "two co ...
. A change in concentration over a distance is called a concentration gradient, a change in pressure over a distance is called a pressure gradient, and a change in temperature over a distance is called a temperature gradient
A temperature gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location. The temperature spatial gradient is a vector quantity with Dimensional analysis, ...
.
The word ''diffusion'' derives from the Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
word, ''diffundere'', which means "to spread out".
A distinguishing feature of diffusion is that it depends on particle random walk
In mathematics, a random walk, sometimes known as a drunkard's walk, is a stochastic process that describes a path that consists of a succession of random steps on some Space (mathematics), mathematical space.
An elementary example of a rand ...
, and results in mixing or mass transport without requiring directed bulk motion. Bulk motion, or bulk flow, is the characteristic of advection
In the fields of physics, engineering, and earth sciences, advection is the transport of a substance or quantity by bulk motion of a fluid. The properties of that substance are carried with it. Generally the majority of the advected substance is a ...
. The term convection
Convection is single or Multiphase flow, multiphase fluid flow that occurs Spontaneous process, spontaneously through the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoy ...
is used to describe the combination of both transport phenomena
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mec ...
.
If a diffusion process can be described by Fick's laws, it is called a normal diffusion (or Fickian diffusion); Otherwise, it is called an anomalous diffusion (or non-Fickian diffusion).
When talking about the extent of diffusion, two length scales are used in two different scenarios:
# Brownian motion of an impulsive point source (for example, one single spray of perfume)—the square root of the mean squared displacement from this point. In Fickian diffusion, this is , where is the dimension
In physics and mathematics, the dimension of a mathematical space (or object) is informally defined as the minimum number of coordinates needed to specify any point within it. Thus, a line has a dimension of one (1D) because only one coo ...
of this Brownian motion;
# Constant concentration source in one dimension—the diffusion length. In Fickian diffusion, this is .
Diffusion vs. bulk flow
"Bulk flow" is the movement/flow of an entire body due to a pressure gradient (for example, water coming out of a tap). "Diffusion" is the gradual movement/dispersion of concentration within a body with no net movement of matter. An example of a process where both bulk motion and diffusion occur is human breathing. First, there is a "bulk flow" process. Thelungs
The lungs are the primary organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the backbone on either side of the heart. Their function in the respiratory syste ...
are located in the thoracic cavity, which expands as the first step in external respiration. This expansion leads to an increase in volume of the alveoli in the lungs, which causes a decrease in pressure in the alveoli. This creates a pressure gradient between the air outside the body at relatively high pressure and the alveoli at relatively low pressure. The air moves down the pressure gradient through the airways of the lungs and into the alveoli until the pressure of the air and that in the alveoli are equal, that is, the movement of air by bulk flow stops once there is no longer a pressure gradient.
Second, there is a "diffusion" process. The air arriving in the alveoli has a higher concentration of oxygen than the "stale" air in the alveoli. The increase in oxygen concentration creates a concentration gradient for oxygen between the air in the alveoli and the blood in the capillaries that surround the alveoli. Oxygen then moves by diffusion, down the concentration gradient, into the blood. The other consequence of the air arriving in alveoli is that the concentration of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
in the alveoli decreases. This creates a concentration gradient for carbon dioxide to diffuse from the blood into the alveoli, as fresh air has a very low concentration of carbon dioxide compared to the blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.
Blood is com ...
in the body.
Third, there is another "bulk flow" process. The pumping action of the heart
The heart is a muscular Organ (biology), organ found in humans and other animals. This organ pumps blood through the blood vessels. The heart and blood vessels together make the circulatory system. The pumped blood carries oxygen and nutrie ...
then transports the blood around the body. As the left ventricle of the heart contracts, the volume decreases, which increases the pressure in the ventricle. This creates a pressure gradient between the heart and the capillaries, and blood moves through blood vessel
Blood vessels are the tubular structures of a circulatory system that transport blood throughout many Animal, animals’ bodies. Blood vessels transport blood cells, nutrients, and oxygen to most of the Tissue (biology), tissues of a Body (bi ...
s by bulk flow down the pressure gradient.
Diffusion in the context of different disciplines
 There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the ''
There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the ''random walk
In mathematics, a random walk, sometimes known as a drunkard's walk, is a stochastic process that describes a path that consists of a succession of random steps on some Space (mathematics), mathematical space.
An elementary example of a rand ...
of the diffusing particles''.
In the phenomenological approach, ''diffusion is the movement of a substance from a region of high concentration to a region of low concentration without bulk motion''. According to Fick's laws, the diffusion flux is proportional to the negative gradient
In vector calculus, the gradient of a scalar-valued differentiable function f of several variables is the vector field (or vector-valued function) \nabla f whose value at a point p gives the direction and the rate of fastest increase. The g ...
of concentrations. It goes from regions of higher concentration to regions of lower concentration. Sometime later, various generalizations of Fick's laws were developed in the frame of thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
and non-equilibrium thermodynamics.
From the ''atomistic point of view'', diffusion is considered as a result of the random walk of the diffusing particles. In molecular diffusion
Molecular diffusion is the motion of atoms, molecules, or other particles of a gas or liquid at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid, size and density (or their product, ...
, the moving molecules in a gas, liquid, or solid are self-propelled by kinetic energy. Random walk of small particles in suspension in a fluid was discovered in 1827 by Robert Brown Robert Brown may refer to: Robert Brown (born 1965), British Director, Animator and author
Entertainers and artists
* Washboard Sam or Robert Brown (1910–1966), American musician and singer
* Robert W. Brown (1917–2009), American printmaker ...
, who found that minute particle suspended in a liquid medium and just large enough to be visible under an optical microscope exhibit a rapid and continually irregular motion of particles known as Brownian movement. The theory of the Brownian motion and the atomistic backgrounds of diffusion were developed by Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
.
The concept of diffusion is typically applied to any subject matter involving random walks in ensembles of individuals.
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
and materials science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials sci ...
, diffusion also refers to the movement of fluid molecules in porous solids. Different types of diffusion are distinguished in porous solids. Molecular diffusion
Molecular diffusion is the motion of atoms, molecules, or other particles of a gas or liquid at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid, size and density (or their product, ...
occurs when the collision with another molecule is more likely than the collision with the pore walls. Under such conditions, the diffusivity is similar to that in a non-confined space and is proportional to the mean free path. Knudsen diffusion occurs when the pore diameter is comparable to or smaller than the mean free path of the molecule diffusing through the pore. Under this condition, the collision with the pore walls becomes gradually more likely and the diffusivity is lower. Finally there is configurational diffusion, which happens if the molecules have comparable size to that of the pore. Under this condition, the diffusivity is much lower compared to molecular diffusion and small differences in the kinetic diameter of the molecule cause large differences in diffusivity.
Biologists often use the terms "net movement" or "net diffusion" to describe the movement of ions or molecules by diffusion. For example, oxygen can diffuse through cell membranes so long as there is a higher concentration of oxygen outside the cell. However, because the movement of molecules is random, occasionally oxygen molecules move out of the cell (against the concentration gradient). Because there are more oxygen molecules outside the cell, the probability
Probability is a branch of mathematics and statistics concerning events and numerical descriptions of how likely they are to occur. The probability of an event is a number between 0 and 1; the larger the probability, the more likely an e ...
that oxygen molecules will enter the cell is higher than the probability that oxygen molecules will leave the cell. Therefore, the "net" movement of oxygen molecules (the difference between the number of molecules either entering or leaving the cell) is into the cell. In other words, there is a ''net movement'' of oxygen molecules down the concentration gradient.
In astronomy
Astronomy is a natural science that studies celestial objects and the phenomena that occur in the cosmos. It uses mathematics, physics, and chemistry in order to explain their origin and their overall evolution. Objects of interest includ ...
, atomic diffusion is used to model the stellar atmospheres of chemically peculiar stars. Diffusion of the elements is critical in understanding the surface composition of degenerate white dwarf
A white dwarf is a Compact star, stellar core remnant composed mostly of electron-degenerate matter. A white dwarf is very density, dense: in an Earth sized volume, it packs a mass that is comparable to the Sun. No nuclear fusion takes place i ...
stars and their evolution over time.
History of diffusion in physics
In the scope of time, diffusion in solids was used long before the theory of diffusion was created. For example,Pliny the Elder
Gaius Plinius Secundus (AD 23/24 79), known in English as Pliny the Elder ( ), was a Roman Empire, Roman author, Natural history, naturalist, and naval and army commander of the early Roman Empire, and a friend of the Roman emperor, emperor Vesp ...
had previously described the cementation process, which produces steel from the element iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
(Fe) through carbon diffusion. Another example is well known for many centuries, the diffusion of colors of stained glass
Stained glass refers to coloured glass as a material or art and architectural works created from it. Although it is traditionally made in flat panels and used as windows, the creations of modern stained glass artists also include three-dimensio ...
or earthenware
Earthenware is glazed or unglazed Vitrification#Ceramics, nonvitreous pottery that has normally been fired below . Basic earthenware, often called terracotta, absorbs liquids such as water. However, earthenware can be made impervious to liquids ...
and Chinese ceramics
Chinese ceramics are one of the most significant forms of Chinese art and ceramics globally. They range from construction materials such as bricks and tiles, to hand-built pottery vessels fired in bonfires or kilns, to the sophisticated Chinese ...
.
In modern science, the first systematic experimental study of diffusion was performed by Thomas Graham. He studied diffusion in gases, and the main phenomenon was described by him in 1831–1833:
"...gases of different nature, when brought into contact, do not arrange themselves according to their density, the heaviest undermost, and the lighter uppermost, but they spontaneously diffuse, mutually and equally, through each other, and so remain in the intimate state of mixture for any length of time."The measurements of Graham contributed to
James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
deriving, in 1867, the coefficient of diffusion for CO2 in the air. The error rate is less than 5%.
In 1855, Adolf Fick, the 26-year-old anatomy demonstrator from Zürich, proposed his law of diffusion. He used Graham's research, stating his goal as "the development of a fundamental law, for the operation of diffusion in a single element of space". He asserted a deep analogy between diffusion and conduction of heat or electricity, creating a formalism similar to Fourier's law for heat conduction (1822) and Ohm's law
Ohm's law states that the electric current through a Electrical conductor, conductor between two Node (circuits), points is directly Proportionality (mathematics), proportional to the voltage across the two points. Introducing the constant of ...
for electric current (1827).
Robert Boyle demonstrated diffusion in solids in the 17th century by penetration of zinc into a copper coin. Nevertheless, diffusion in solids was not systematically studied until the second part of the 19th century. William Chandler Roberts-Austen, the well-known British metallurgist and former assistant of Thomas Graham studied systematically solid state diffusion on the example of gold in lead in 1896. :
"... My long connection with Graham's researches made it almost a duty to attempt to extend his work on liquid diffusion to metals."In 1858, Rudolf Clausius introduced the concept of the
mean free path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a ...
. In the same year, James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
developed the first atomistic theory of transport processes in gases. The modern atomistic theory of diffusion and Brownian motion was developed by Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
, Marian Smoluchowski and Jean-Baptiste Perrin. Ludwig Boltzmann
Ludwig Eduard Boltzmann ( ; ; 20 February 1844 – 5 September 1906) was an Austrian mathematician and Theoretical physics, theoretical physicist. His greatest achievements were the development of statistical mechanics and the statistical ex ...
, in the development of the atomistic backgrounds of the macroscopic transport processes, introduced the Boltzmann equation, which has served mathematics and physics with a source of transport process ideas and concerns for more than 140 years.S. Chapman, T. G. Cowling (1970) ''The Mathematical Theory of Non-uniform Gases: An Account of the Kinetic Theory of Viscosity, Thermal Conduction and Diffusion in Gases'', Cambridge University Press (3rd edition), .
In 1920–1921, George de Hevesy measured self-diffusion using radioisotopes. He studied self-diffusion of radioactive isotopes of lead in the liquid and solid lead.
Yakov Frenkel (sometimes, Jakov/Jacob Frenkel) proposed, and elaborated in 1926, the idea of diffusion in crystals through local defects (vacancies and interstitial atoms). He concluded, the diffusion process in condensed matter is an ensemble of elementary jumps and quasichemical interactions of particles and defects. He introduced several mechanisms of diffusion and found rate constants from experimental data.
Sometime later, Carl Wagner and Walter H. Schottky developed Frenkel's ideas about mechanisms of diffusion further. Presently, it is universally recognized that atomic defects are necessary to mediate diffusion in crystals.
Henry Eyring, with co-authors, applied his theory of absolute reaction rates to Frenkel's quasichemical model of diffusion. The analogy between reaction kinetics and diffusion leads to various nonlinear versions of Fick's law.
Basic models of diffusion
Definition of diffusion flux
Each model of diffusion expresses the diffusion flux with the use of concentrations, densities and their derivatives. Flux is a vector representing the quantity and direction of transfer. Given a smallarea
Area is the measure of a region's size on a surface. The area of a plane region or ''plane area'' refers to the area of a shape or planar lamina, while '' surface area'' refers to the area of an open surface or the boundary of a three-di ...
with normal , the transfer of a physical quantity
A physical quantity (or simply quantity) is a property of a material or system that can be Quantification (science), quantified by measurement. A physical quantity can be expressed as a ''value'', which is the algebraic multiplication of a ''nu ...
through the area per time is
:
where is the inner product
In mathematics, an inner product space (or, rarely, a Hausdorff pre-Hilbert space) is a real vector space or a complex vector space with an operation called an inner product. The inner product of two vectors in the space is a scalar, ofte ...
and is the little-o notation. If we use the notation of vector area then
:
The dimension
In physics and mathematics, the dimension of a mathematical space (or object) is informally defined as the minimum number of coordinates needed to specify any point within it. Thus, a line has a dimension of one (1D) because only one coo ...
of the diffusion flux is luxnbsp;= uantity( ime� rea. The diffusing physical quantity may be the number of particles, mass, energy, electric charge, or any other scalar extensive quantity. For its density, , the diffusion equation has the form
:
where is intensity of any local source of this quantity (for example, the rate of a chemical reaction).
For the diffusion equation, the no-flux boundary conditions can be formulated as on the boundary, where is the normal to the boundary at point .
Normal single component concentration gradient
Fick's first law: The diffusion flux, , is proportional to the negative gradient of spatial concentration, : : where ''D'' is the diffusion coefficient, which can be estimated for a given mixture using, for example, the empirical Vignes correlation model or the physically-motivated entropy scaling. The corresponding diffusion equation (Fick's second law) is : In case the diffusion coefficient is independent of , Fick's second law can be simplified to : where is theLaplace operator
In mathematics, the Laplace operator or Laplacian is a differential operator given by the divergence of the gradient of a Scalar field, scalar function on Euclidean space. It is usually denoted by the symbols \nabla\cdot\nabla, \nabla^2 (where \ ...
,
:
Multicomponent diffusion and thermodiffusion
Fick's law describes diffusion of an admixture in a medium. The concentration of this admixture should be small and the gradient of this concentration should be also small. The driving force of diffusion in Fick's law is the antigradient of concentration, . In 1931, Lars Onsager included the multicomponent transport processes in the general context of linear non-equilibrium thermodynamics. For multi-component transport, : where is the flux of the th physical quantity (component), is the th thermodynamic force and is Onsager's matrix of ''kinetic transport coefficients''. The thermodynamic forces for the transport processes were introduced by Onsager as the space gradients of the derivatives of theentropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
density (he used the term "force" in quotation marks or "driving force"):
:
where are the "thermodynamic coordinates".
For the heat and mass transfer one can take (the density of internal energy) and is the concentration of the th component. The corresponding driving forces are the space vectors
: because
where ''T'' is the absolute temperature and is the chemical potential of the th component. It should be stressed that the separate diffusion equations describe the mixing or mass transport without bulk motion. Therefore, the terms with variation of the total pressure are neglected. It is possible for diffusion of small admixtures and for small gradients.
For the linear Onsager equations, we must take the thermodynamic forces in the linear approximation near equilibrium:
:
where the derivatives of are calculated at equilibrium .
The matrix of the ''kinetic coefficients'' should be symmetric ( Onsager reciprocal relations) and positive definite ( for the entropy growth).
The transport equations are
:
Here, all the indexes are related to the internal energy (0) and various components. The expression in the square brackets is the matrix of the diffusion (''i'',''k'' > 0), thermodiffusion (''i'' > 0, ''k'' = 0 or ''k'' > 0, ''i'' = 0) and thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
() coefficients.
Under isothermal conditions ''T'' = constant. The relevant thermodynamic potential
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
is the free energy (or the free entropy
Free may refer to:
Concept
* Freedom, the ability to act or change without constraint or restriction
* Emancipate, attaining civil and political rights or equality
* Free (''gratis''), free of charge
* Gratis versus libre, the difference bet ...
). The thermodynamic driving forces for the isothermal diffusion are antigradients of chemical potentials, , and the matrix of diffusion coefficients is
:
(''i,k'' > 0).
There is intrinsic arbitrariness in the definition of the thermodynamic forces and kinetic coefficients because they are not measurable separately and only their combinations can be measured. For example, in the original work of Onsager the thermodynamic forces include additional multiplier ''T'', whereas in the Course of Theoretical Physics this multiplier is omitted but the sign of the thermodynamic forces is opposite. All these changes are supplemented by the corresponding changes in the coefficients and do not affect the measurable quantities.
Nondiagonal diffusion must be nonlinear
The formalism of linear irreversible thermodynamics (Onsager) generates the systems of linear diffusion equations in the form : If the matrix of diffusion coefficients is diagonal, then this system of equations is just a collection of decoupled Fick's equations for various components. Assume that diffusion is non-diagonal, for example, , and consider the state with . At this state, . If at some points, then becomes negative at these points in a short time. Therefore, linear non-diagonal diffusion does not preserve positivity of concentrations. Non-diagonal equations of multicomponent diffusion must be non-linear.Applied forces
TheEinstein relation (kinetic theory)
In physics (specifically, the kinetic theory of gases), the Einstein relation is a previously unexpected connection revealed independently by William Sutherland in 1904, Albert Einstein in 1905, and by Marian Smoluchowski in 1906 in their works ...
connects the diffusion coefficient and the mobility (the ratio of the particle's terminal drift velocity to an applied force
In physics, a force is an influence that can cause an Physical object, object to change its velocity unless counterbalanced by other forces. In mechanics, force makes ideas like 'pushing' or 'pulling' mathematically precise. Because the Magnitu ...
). For charged particles:
:
where ''D'' is the diffusion constant, ''μ'' is the "mobility", ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
, ''T'' is the absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
, and ''q'' is the elementary charge
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, ...
, that is, the charge of one electron.
Below, to combine in the same formula the chemical potential ''μ'' and the mobility, we use for mobility the notation .
Diffusion across a membrane
The mobility-based approach was further applied by T. Teorell. In 1935, he studied the diffusion of ions through a membrane. He formulated the essence of his approach in the formula: :the flux is equal to mobility × concentration × force per gram-ion. This is the so-called ''Teorell formula''. The term "gram-ion" ("gram-particle") is used for a quantity of a substance that contains the Avogadro number of ions (particles). The common modern term is mole. The force under isothermal conditions consists of two parts: # Diffusion force caused by concentration gradient: . # Electrostatic force caused by electric potential gradient: . Here ''R'' is the gas constant, ''T'' is the absolute temperature, ''n'' is the concentration, the equilibrium concentration is marked by a superscript "eq", ''q'' is the charge and ''φ'' is the electric potential. The simple but crucial difference between the Teorell formula and the Onsager laws is the concentration factor in the Teorell expression for the flux. In the Einstein–Teorell approach, if for the finite force the concentration tends to zero then the flux also tends to zero, whereas the Onsager equations violate this simple and physically obvious rule. The general formulation of the Teorell formula for non-perfect systems under isothermal conditions is : where ''μ'' is thechemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
, ''μ''0 is the standard value of the chemical potential.
The expression is the so-called activity. It measures the "effective concentration" of a species in a non-ideal mixture. In this notation, the Teorell formula for the flux has a very simple form
:
The standard derivation of the activity includes a normalization factor and for small concentrations , where is the standard concentration. Therefore, this formula for the flux describes the flux of the normalized dimensionless quantity :
:
Ballistic time scale
The Einstein model neglects the inertia of the diffusing partial. The alternativeLangevin equation
In physics, a Langevin equation (named after Paul Langevin) is a stochastic differential equation describing how a system evolves when subjected to a combination of deterministic and fluctuating ("random") forces. The dependent variables in a Lange ...
starts with Newton's second law of motion:
:
where
* ''x'' is the position.
* ''μ'' is the mobility of the particle in the fluid or gas, which can be calculated using the Einstein relation (kinetic theory)
In physics (specifically, the kinetic theory of gases), the Einstein relation is a previously unexpected connection revealed independently by William Sutherland in 1904, Albert Einstein in 1905, and by Marian Smoluchowski in 1906 in their works ...
.
* ''m'' is the mass of the particle.
* ''F'' is the random force applied to the particle.
* ''t'' is time.
Solving this equation, one obtained the time-dependent diffusion constant in the long-time limit and when the particle is significantly denser than the surrounding fluid,
:
where
* ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
;
* ''T'' is the absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
.
* ''μ'' is the mobility of the particle in the fluid or gas, which can be calculated using the Einstein relation (kinetic theory)
In physics (specifically, the kinetic theory of gases), the Einstein relation is a previously unexpected connection revealed independently by William Sutherland in 1904, Albert Einstein in 1905, and by Marian Smoluchowski in 1906 in their works ...
.
* ''m'' is the mass of the particle.
* ''t'' is time.
At long time scales, Einstein's result is recovered, but short time scales, the ''ballistic regime'' are also explained. Moreover, unlike the Einstein approach, a velocity can be defined, leading to the Fluctuation-dissipation theorem, connecting the competition between friction and random forces in defining the temperature.
Jumps on the surface and in solids
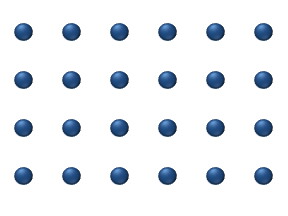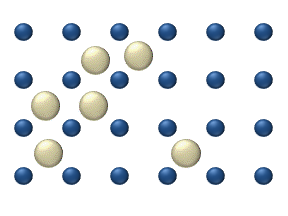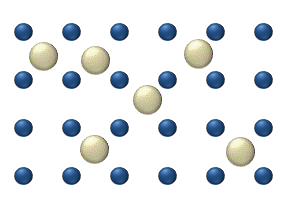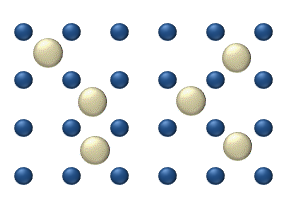 Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
:
Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
: