|
Transport Coefficient
A transport coefficient \gamma measures how rapidly a perturbed system returns to equilibrium. The transport coefficients occur in transport phenomenon with transport laws : \mathbf_k = \gamma_k \mathbf_k where: : \mathbf_k is a flux of the property k : the transport coefficient \gamma _k of this property k : \mathbf_k, the gradient force which acts on the property k . Transport coefficients can be expressed via a Green–Kubo relation: :\gamma = \int_0^\infty \left\langle \dot(t) \dot(0) \right\rangle \, dt, where A is an observable occurring in a perturbed Hamiltonian, \langle \cdot \rangle is an ensemble average and the dot above the ''A'' denotes the time derivative.Water in Biology, Chemistry, and Physics: Experimental Overviews and Computational Methodologies, G. Wilse Robinson, , p. 80Google Books/ref> For times t that are greater than the correlation time of the fluctuations of the observable the transport coefficient obeys a generalized Einstein relation: :2t\ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transport Phenomenon
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mechanics and thermodynamics, it places a heavy emphasis on the commonalities between the topics covered. Mass, momentum, and heat transport all share a very similar mathematical framework, and the parallels between them are exploited in the study of transport phenomena to draw deep mathematical connections that often provide very useful tools in the analysis of one field that are directly derived from the others. The fundamental analysis in all three subfields of mass, heat, and momentum transfer are often grounded in the simple principle that the total sum of the quantities being studied must be conserved by the system and its environment. Thus, the different phenomena that lead to transport are each considered individually with the knowled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green–Kubo Relations
The Green–Kubo relations ( Melville S. Green 1954, Ryogo Kubo 1957) give the exact mathematical expression for transport coefficients \gamma in terms of integrals of time correlation functions: :\gamma = \int_0^\infty \left\langle \dot(t) \dot(0) \right\rangle \;t. Thermal and mechanical transport processes Thermodynamic systems may be prevented from relaxing to equilibrium because of the application of a field (e.g. electric or magnetic field), or because the boundaries of the system are in relative motion (shear) or maintained at different temperatures, etc. This generates two classes of nonequilibrium system: mechanical nonequilibrium systems and thermal nonequilibrium systems. The standard example of an electrical transport process is Ohm's law, which states that, at least for sufficiently small applied voltages, the current ''I'' is linearly proportional to the applied voltage ''V'', : I = \sigma V.\, As the applied voltage increases one expects to see deviations from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
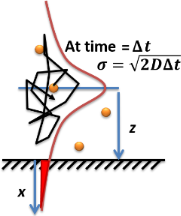
Einstein Relation (kinetic Theory)
In physics (specifically, the kinetic theory of gases), the Einstein relation is a previously unexpected connection revealed independently by William Sutherland in 1904, Albert Einstein in 1905, and by Marian Smoluchowski in 1906 in their works on Brownian motion. The more general form of the equation is D = \mu \, k_\text T, where * is the diffusion coefficient; * is the "mobility", or the ratio of the particle's terminal drift velocity to an applied force, ; * is the Boltzmann constant; * is the absolute temperature. This equation is an early example of a fluctuation-dissipation relation. Two frequently used important special forms of the relation are: * Einstein–Smoluchowski equation, for diffusion of charged particles: D = \frac * Stokes–Einstein equation, for diffusion of spherical particles through a liquid with low Reynolds number: D = \frac Here * is the electrical charge of a particle; * is the electrical mobility of the charged particle; * is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion Constant
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation. A diffusion process that obeys Fick's laws is called normal or Fickian diffusion; otherwise, it is called anomalous diffusion or non-Fickian diffusion. History In 1855, physiologist Adolf Fick first reported* * his now well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of Thomas Graham, which fell short of proposing the fundamental laws for which Fick would become famous. Fick's law is analogous to the relationships discovered at the same epoch by other eminent scientists: Darcy's law (hydraulic flow), Ohm's law (charge transport), and Fourier's Law (heat transport). Fick's experiments (modeled on Graham's) dealt with measuring the concentrations and f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fick's Laws Of Diffusion
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation. A diffusion process that obeys Fick's laws is called normal or Fickian diffusion; otherwise, it is called anomalous diffusion or non-Fickian diffusion. History In 1855, physiologist Adolf Fick first reported* * his now well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of Thomas Graham, which fell short of proposing the fundamental laws for which Fick would become famous. Fick's law is analogous to the relationships discovered at the same epoch by other eminent scientists: Darcy's law (hydraulic flow), Ohm's law (charge transport), and Fourier's Law (heat transport). Fick's experiments (modeled on Graham's) dealt with measuring the concentration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Rockwool or Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla T is the temperature gradient. This is known as Fourier's Law for heat conduction. Although commonly expressed as a scalar, the most general form of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fourier's Law
Conduction is the process by which heat is transferred from the hotter end to the colder end of an object. The ability of the object to conduct heat is known as its ''thermal conductivity'', and is denoted . Heat spontaneously flows along a temperature gradient (i.e. from a hotter body to a colder body). For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becoming more uniform. In conduction, the heat flow is within and through the body itself. In contrast, in heat transfer by thermal radiation, the transfer is often between bodies, which may be separated spatially. Heat can also be transferred by a combination of conduction and radiation. In solids, conduction is mediated by the combination of vibrations and collisions of mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Conductivity (solid State)
Ionic conductivity (denoted by ) is a measure of a substance's tendency towards ionic conduction. Ionic conduction is the movement of ions. The phenomenon is observed in solids and solutions. Ionic conduction is one mechanism of current. In crystalline solids In most solids, ions rigidly occupy fixed positions, strongly embraced by neighboring atoms or ions. In some solids, selected ions are highly mobile allowing ionic conduction. The mobility increases with temperature. Materials exhibiting this property are used in batteries. A well-known ion conductive solid is β''-alumina ("BASE"), a form of aluminium oxide that has channels through which sodium cations can hop. When this ceramic is complexed with a mobile ion, such as Na+, it behaves as so-called fast ion conductor. BASE is used as a membrane in several types of molten salt electrochemical cell. In glasses Ion conduction in disordered solids like glasses, polymers, nanocomposites, defective crystals and other d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Transfer Coefficient
In engineering, the mass transfer coefficient is a diffusion rate constant that relates the mass transfer rate, mass transfer area, and concentration change as driving force: k_c = \frac Where: *k_c is the mass transfer coefficient ol/(s·m2)/(mol/m3) or m/s *\dot_A is the mass transfer rate ol/s*A is the effective mass transfer area 2*\Delta c_A is the driving force concentration difference ol/m3 This can be used to quantify the mass transfer between phases, immiscible and partially miscible fluid mixtures (or between a fluid and a porous solide.g.: during adsorption process.). Quantifying mass transfer allows for design and manufacture of separation process equipment that can meet specified requirements, estimate what will happen in real life situations (chemical spill), etc. Mass transfer coefficients can be estimated from many different theoretical equations, correlations, and analogies that are functions of material properties, intensive properties and flow regime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shear Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscous Stress Tensor
The viscous stress tensor is a tensor used in continuum mechanics to model the part of the stress at a point within some material that can be attributed to the strain rate, the rate at which it is deforming around that point. The viscous stress tensor is formally similar to the elastic stress tensor (Cauchy tensor) that describes internal forces in an elastic material due to its deformation. Both tensors map the normal vector of a surface element to the density and direction of the stress acting on that surface element. However, elastic stress is due to the ''amount'' of deformation ( strain), while viscous stress is due to the ''rate'' of change of deformation over time (strain rate). In viscoelastic materials, whose behavior is intermediate between those of liquids and solids, the total stress tensor comprises both viscous and elastic ("static") components. For a completely fluid material, the elastic term reduces to the hydrostatic pressure. In an arbitrary coordinate syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Newtonian Fluid
A Newtonian fluid is a fluid in which the viscous stress tensor, viscous stresses arising from its Fluid dynamics, flow are at every point linearly correlated to the local strain rate — the derivative (mathematics), rate of change of its deformation (mechanics), deformation over time. Stresses are proportional to the rate of change of the fluid's velocity, velocity vector. A fluid is Newtonian only if the tensors that describe the viscous stress and the strain rate are related by a constant viscosity, viscosity tensor that does not depend on the stress state and velocity of the flow. If the fluid is also isotropic (mechanical properties are the same along any direction), the viscosity tensor reduces to two real coefficients, describing the fluid's resistance to continuous Shearing (physics), shear deformation and continuous compression (physical), compression or expansion, respectively. Newtonian fluids are the simplest mathematical models of fluids that account for viscosity. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |