Dethioacetylated on:
[Wikipedia]
[Google]
[Amazon]
In
RSNa + R'COCl -> R'COSR + NaCl
Another common route entails the displacement of CH3COSK + RX -> CH3COSR + KX
The analogous alkylation of an acetate salt is rarely practiced. The alkylation can be conducted using CH3COSH + R'2NCH2OH -> CH3COSCH2NR'2 + H2O
Thioesters can be prepared by condensation of thiols and carboxylic acids in the presence of dehydrating agents:
:RSH + R'CO2H -> RSC(O)R' + H2O
A typical dehydration agent is DCC. Efforts to improve the sustainability of thioester synthesis have also been reported utilising safer coupling reagent T3P and greener solvent
 In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the
In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, thioesters are organosulfur compounds
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur ...
with the functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
. They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by the ''thio-
The prefix thio-, when applied to a chemical, such as an ion, means that an oxygen atom in the compound has been replaced by a sulfur atom. This term is often used in organic chemistry. For example, from the word ''ether,'' referring to an oxyg ...
'' prefix. They are the product of esterification between a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
() and a thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
(). In biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, the best-known thioesters are derivatives of coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a subs ...
, e.g., acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764.
Synthesis
The most typical route to thioester involves the reaction of an acid chloride with analkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
salt of a thiol:
:halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s by the alkali metal salt of a thiocarboxylic acid
In organic chemistry, thiocarboxylic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a Thioketone, thione form () and a thiol form (). These ar ...
. For example, thioacetate esters are commonly prepared by alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
of potassium thioacetate
Potassium thioacetate is an organosulfur compound and a salt with the formula . This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives.
Synthesis and reactions
Potassium thioacetate, which is ...
:
:Mannich base A Mannich base is a beta-amino-ketone, which is formed in the reaction of an amine, formaldehyde (or an aldehyde) and a carbon acid. The Mannich base is an endproduct in the Mannich reaction, which is nucleophilic addition reaction of a non-enoliz ...
s and the thiocarboxylic acid:
:cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclopenta ...
. Acid anhydrides and some lactones also give thioesters upon treatment with thiols in the presence of a base.
Thioesters can be conveniently prepared from alcohols by the Mitsunobu reaction, using thioacetic acid
Thioacetic acid is an organosulfur compound with the molecular formula . It is the sulfur analogue of acetic acid (), as implied by the ''thio-'' prefix. It is a yellow liquid with a strong thiol-like odor. It is used in organic synthesis for the ...
.
They also arise via carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbon ...
of alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s and alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s in the presence of thiols.
Reactions
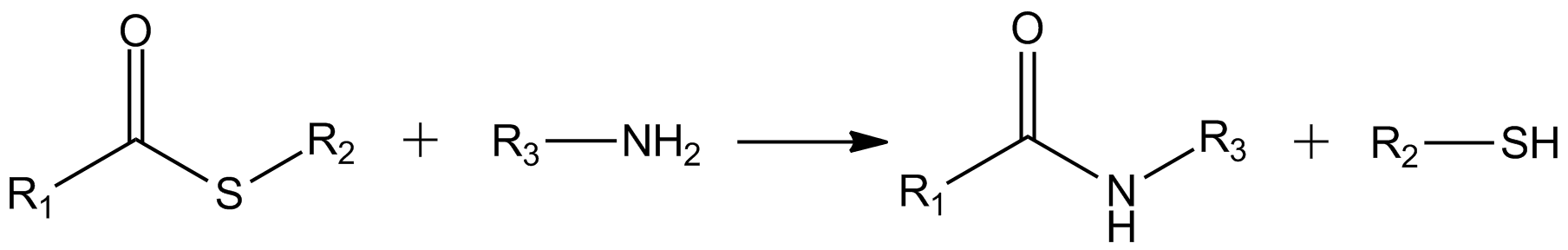
Thioesters hydrolyze to thiols and the carboxylic acid: :RC(O)SR' + H2O → RCO2H + RSH The carbonyl center in thioesters is more reactive toward amine nucleophiles to giveamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
s:
: In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the
In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the Fukuyama coupling
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998. Adv ...
, in which the thioester is coupled with an organozinc halide
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compoun ...
by a palladium catalyst to give a ketone.
:
Biochemistry
Thioesters are common intermediates in many biosynthetic reactions, including the formation and degradation offatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, fr ...
s and mevalonate, precursor to steroids. Examples include malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Functions
It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Fatty acid biosynthesis
Malonyl-CoA provides 2-carbon units to fatty acids and commi ...
, acetoacetyl-CoA
Acetoacetyl CoA is the precursor of HMG-CoA in the mevalonate pathway, which is essential for cholesterol biosynthesis. It also takes a similar role in the ketone bodies synthesis (ketogenesis) pathway of the liver. In the ketone bodies digestion ...
, propionyl-CoA, cinnamoyl-CoA
Cinnamoyl-Coenzyme A is an intermediate in the phenylpropanoids metabolic pathway.
Enzymes using Cinnamoyl-Coenzyme A
* Cinnamoyl-CoA reductase, an enzyme that catalyzes the chemical reaction cinnamaldehyde + CoA + NADP+ → cinnamoyl-CoA + NADP ...
, and acyl carrier protein (ACP) thioesters. Acetogenesis Acetogenesis is a process through which acetate is produced either by the reduction of CO2 or by the reduction of organic acids, rather than by the oxidative breakdown of carbohydrates or ethanol, as with acetic acid bacteria.
The different bacte ...
proceeds via the formation of acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
. The biosynthesis of lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity ...
, which comprises a large fraction of the Earth's land biomass, proceeds via a thioester derivative of caffeic acid. These thioesters arise analogously to those prepared synthetically, the difference being that the dehydration agent is ATP. In addition, thioesters play an important role in the tagging of proteins with ubiquitin, which tags the protein for degradation.
Oxidation of the sulfur atom in thioesters (thiolactones) is postulated in the bioactivation of the antithrombotic prodrugs ticlopidine, clopidogrel, and prasugrel.
Thioesters and the origin of life
As posited in a "Thioester World", thioesters are possible precursors to life. As Christian de Duve explains:It is revealing that thioesters are obligatory intermediates in several key processes in whichHowever, due to the high free energy change of thioester's hydrolysis and correspondingly their low equilibrium constants, it is unlikely that these compounds could have accumulated abiotically to any significant extent especially in hydrothermal vent conditions.ATP ATP may refer to: Companies and organizations * Association of Tennis Professionals, men's professional tennis governing body * American Technical Publishers, employee-owned publishing company * ', a Danish pension * Armenia Tree Project, non ...is either used or regenerated. Thioesters are involved in the synthesis of all esters, including those found in complexlipid Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include ...s. They also participate in the synthesis of a number of other cellular components, includingpeptide Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A ...s,fatty acid In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, fr ...s,sterol Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the go ...s,terpene Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ar ...s, porphyrins, and others. In addition, thioesters are formed as key intermediates in several particularly ancient processes that result in the assembly of ATP. In both these instances, the thioester is closer than ATP to the process that uses or yields energy. In other words, thioesters could have actually played the role of ATP in a "thioester world" initially devoid of ATP. Eventually, hesethioesters could have served to usher in ATP through its ability to support the formation of bonds between phosphate groups.
Thionoesters
Thionoesters are isomeric with thioesters. In a thionoester, sulfur replaces the carbonyl oxygen in an ester. Methyl thionobenzoate is C6H5C(S)OCH3. Such compounds are typically prepared by the reaction of thethioacyl chloride
In organic chemistry, thioacyl chloride is a functional group of the type RC(S)Cl, where R is an organic substituent. Thioacyl chlorides are analogous to acid chlorides, but much rarer and less robust. The best studied is thiobenzoyl chloride, a ...
with an alcohol.
They can also be made by the reaction of Lawesson's reagent
Lawesson's reagent (LR) is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a sys ...
with esters or by treating pinner salts with hydrogen sulphide. An alternatively, various thionoesters may be prepared through the transesterification of an existing methyl thionoester with an alcohol under base-catalyzed conditions.
See also
*Thiocarboxylic acid
In organic chemistry, thiocarboxylic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a Thioketone, thione form () and a thiol form (). These ar ...
* Liebeskind-Srogl coupling
* Aldrithiol-2
References
{{Functional group Functional groups Origin of life