Camptothecin on:
[Wikipedia]
[Google]
[Amazon]
Camptothecin (CPT) is a

 Studies have shown that substitution at position 7, 9, 10 and 11 can have positive effect on CPT activity and physical properties, e.g. potency and metabolic stability. Enlargement of the lactone ring by one unit also enhances its abilities, as in homocamptothecin. Substitution at position 12 and 14 leads to inactive derivative.
Studies have shown that substitution at position 7, 9, 10 and 11 can have positive effect on CPT activity and physical properties, e.g. potency and metabolic stability. Enlargement of the lactone ring by one unit also enhances its abilities, as in homocamptothecin. Substitution at position 12 and 14 leads to inactive derivative.

CPT is linked to a

 Like all other monoterpenoid indole-alkaloids, biosynthesis of camptothecin requires production of the
Like all other monoterpenoid indole-alkaloids, biosynthesis of camptothecin requires production of the
topoisomerase inhibitor
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular rep ...
. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical sy ...
for anticancer drugs. It was isolated from the bark and stem of ''Camptotheca acuminata
''Camptotheca'' (happy tree, cancer tree, or tree of life) is a genus of medium-sized deciduous trees growing to tall, native to southern China and Tibet. The genus is usually included in the tupelo family Nyssaceae, but sometimes included (wi ...
'' (Camptotheca, Happy tree), a tree
In botany, a tree is a perennial plant with an elongated stem, or trunk, usually supporting branches and leaves. In some usages, the definition of a tree may be narrower, including only woody plants with secondary growth, plants that are ...
native to China used in traditional Chinese medicine
Traditional Chinese medicine (TCM) is an alternative medical practice drawn from traditional medicine in China. It has been described as "fraught with pseudoscience", with the majority of its treatments having no logical mechanism of acti ...
. It has been used clinically more recently in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
, with good results. Four CPT analogues have been approved and are used in cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
chemotherapy
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs ( chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemothe ...
today, topotecan
Topotecan, sold under the brand name Hycamtin among others, is a chemotherapeutic agent medication that is a topoisomerase inhibitor. It is a synthetic, water-soluble analog of the natural chemical compound camptothecin. It is used in the form o ...
, irinotecan
Irinotecan, sold under the brand name Camptosar among others, is a medication used to treat colon cancer, and small cell lung cancer. For colon cancer it is used either alone or with fluorouracil. For small cell lung cancer it is used with cisp ...
, belotecan
Belotecan is a drug used in chemotherapy. It is a semi-synthetic camptothecin analogue indicated for small-cell lung cancer and ovarian cancer
Ovarian cancer is a cancerous tumor of an ovary. It may originate from the ovary itself or more co ...
, and trastuzumab deruxtecan
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan (a derivative of ...
. Camptothecin has also been found in other plants including ''Chonemorpha fragrans
''Chonemorpha fragrans'', the frangipani vine or climbing frangipani, is a plant species in the genus '' Chonemorpha''. It is a vigorous, generally evergreen, climbing shrub producing stems or more long that can climb to the tops of the tallest ...
''.
Structures
CPT has a planar pentacyclic ring structure, that includes a pyrrolo ,4-βquinoline moiety (rings A, B and C), conjugated pyridone moiety (ring D) and onechiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
center at position 20 within the alpha- hydroxy lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
ring with (S) configuration (the E-ring). Its planar structure is thought to be one of the most important factors in topoisomerase inhibition.
Binding
CPT binds to the topoisomerase I and DNA complex (the covalent complex) resulting in a ternary complex, and thereby stabilizing it. This prevents DNA re-ligation and therefore causes DNA damage which results in apoptosis. CPT binds both to the enzyme and DNA with hydrogen bonds. The most important part of the structure is the E-ring which interacts from three different positions with the enzyme. The hydroxyl group in position 20 forms hydrogen bond to the side chain on aspartic acid number533
__NOTOC__
Year 533 ( DXXXIII) was a common year starting on Saturday (link will display the full calendar) of the Julian calendar. At the time, it was known as the Year of the Consulship of Iustinianus without colleague (or, less frequently, y ...
(Asp533) in the enzyme. It is critical that the configuration of the chiral carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
is (S) because (R) is inactive. The lactone is bonded with two hydrogen bonds to the amino
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
groups on arginine 364 (Arg364).
The D-ring interacts with the +1 cytosine
Cytosine () ( symbol C or Cyt) is one of the four nucleobases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an ...
on non-cleaved strand and stabilizes the topoisomerase I-DNA covalent complex by forming hydrogen bond. This hydrogen bond is between carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
group in position 17 on the D-ring and amino group on the pyrimidine ring of +1 cytosine. CPT is selectively cytotoxic to the cells replicating DNA during S phase and its toxicity is primarily a result of conversion of single-strand breaks into double-strand breaks when the replication fork collides with the cleavage complexes formed by DNA and CPT.
Chemistry
The lactone ring in CPT is highly susceptible tohydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
. The open ring form is inactive and it must therefore be closed to inhibit topoisomerase I. The closed form is favored in acidic condition, as it is in many cancer cells microenvironment.
CPT is transported into the cell by passive diffusion
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to dri ...
. Cellular uptake is favored by lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lip ...
, which enhances intracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions ...
accumulation.
Lipophilicity makes compounds more stable because of improved lactone partitioning into red blood cells and consequently less hydrolysis of the lactone.
CPT has affinity for human serum albumin (HSA), especially the carboxylate form of CPT. Because of that, the equilibrium between the lactone ring and the carboxylate form is driven toward the carboxylate. Reduced drug-HSA interactions
Interaction is action that occurs between two or more objects, with broad use in philosophy and the sciences. It may refer to:
Science
* Interaction hypothesis, a theory of second language acquisition
* Interaction (statistics)
* Interactions o ...
could result in improved activity.
Structure-activity relationship
A- and B-ring modification
Alkyl substitution
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
substitution at position 7 has shown increased cytotoxicity, such as ethyl (C2H5) or chloromethyl (CH2Cl). These groups are able to react with the DNA in the presence of topoisomerase I which leads to more tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
activity. It has also been shown that increasing the length of the carbon chain (in position 7) leads to increased lipophilicity and consequently greater potency and stability in human plasma.
Other 7-modified CPT analogues are silatecans and karenitecins. They are potent inhibitors on topoisomerase I and both have alkylsilyl groups in position 7 which make them lipophilic and more stable. Silatecans or 7-silylcampthothecins have shown reduced drug-HSA interactions which contributes to its blood stability and they can also cross the blood brain barrier
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the c ...
. DB-67 is a 10-hydroxy derivative and is among the most active silatecans. BNP1350 which belongs to the series of karenitecins exhibits cytotoxic activity and ability to overcome drug resistance
Drug resistance is the reduction in effectiveness of a medication such as an antimicrobial or an antineoplastic in treating a disease or condition. The term is used in the context of resistance that pathogens or cancers have "acquired", that is ...
. Still another route to make CPT’s lipophilic is to introduce lipophilic substituents, such as iminomethyl or oxyiminomethyl moieties. One of the most potent compounds is the oxyiminomethyl derivative ST1481 that has the advantage to overcome drug resistance caused by transport systems.
Basic nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
in a carbon chain at position 7 makes the compound more hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are ...
and hence more water-soluble. For example, is a derivate called CKD-602, which is a potent topoisomerase I inhibitor and successfully overcomes the poor water solubility and toxicity seen with CPT.
Considerably greater activity can be achieved by putting electron-withdrawing groups like amino, nitro
Nitro may refer to:
Chemistry
*Nitrogen, a chemical element and a gas except at very low temperatures, with which many compounds are formed:
**Nitro compound, an organic compound containing one or more nitro functional groups, -NO2
**Nitroalkene, ...
, bromo or chloro at position 9 and 10 and hydroxyl group at position 10 or 11. But these compounds are relatively insoluble in aqueous solutions, which causes difficulty in administrations. Methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula .
On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position a ...
group at both position 10 and 11 simultaneously leads to inactivity.
Hexacyclic CPT analogues
Hexacyclic CPT analogues have shown great potency. For example,methylenedioxy
Methylenedioxy is the term used in the field of chemistry, particularly in organic chemistry, for a functional group with the structural formula R-O-CH2-O-R' which is connected to the rest of a molecule by two chemical bonds. The methylenedioxy gr ...
or ethylenedioxy group connected between 10 and 11 form a 5 or 6 membered ring which leads to more water-soluble derivates and increased potency. Researches have shown that ethylenedioxy analogues are less potent than methylenedioxy. The reason is the unfavorable steric interactions of ethylenedioxy analogues with the enzyme.
Adding amino or chloro group at 9th position or chloromethyl group at 7th position to these 10, 11-methylenedioxy or ethylenedioxy analogues results in compounds with even greater cytotoxicity but weaker solubility in water. To yield 10, 11-methylenedioxy or ethylenedioxy analogues with good water solubility a good way is to introduce a water solubilising substituent at position 7. Lurtotecan meets those requirements; it’s a 10, 11-ethylenedioxy analogue with a 4-methylpiperazino-methylene at position 7 and has shown a great potency in clinical researches.
A ring can also be formed between position 7 and 9, like position 10 and 11. That gives new opportunities to make water-soluble derivatives These hexacyclic CPT become more active when electron-withdrawing groups are put in position 11 and methyl or amino groups at 10. Exatecan is an example of hexacyclic CPT that has a 6 membered ring over position 7 and 9, and is 10-methyl, 11-fluoro substituted It is water-soluble and more potent than topotecan.
C- and D-ring modification
The C- and D-rings have an essential role in the antitumor activity. Replacement in any position results in much less potent compound than parent compound in other cytotoxicity assay.E-ring modifications
The E-ring doesn’t allow many structural changes without losing CPT activity because it is necessary for binding to the active site of TOP I. One possible replacement is changing the hydroxyl group to Cl, F or Br because theirpolarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, considering that matter is made up of elementar ...
is sufficient to stabilize the enzyme-complex.
Another possible modification is to insert a methylene between hydroxyl and lactone on the E-ring yielding a seven membered β-hydroxylactone group, so-called homocamptothecin (hCPT). The hCPT’s hydroxyl has less inductive effect
In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond.
It is present in a σ (sigma ...
on the carboxyl group which makes the lactone very reactive. This enhances the interaction of the free hydroxyl group optimally with topoisomerase I and the covalent complex that forms in its presence are more stable. The E-ring of hCPT opens more slowly and the opening is irreversible. hCPTs exhibit enhanced human plasma stability because of decreased protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
binding and more affinity for red blood cells than CPT.
CPT analogues
Since the discovery of CPT many analogues have been synthesized. Below is a schematic view of the CPT analogues that have been mentioned in the text above.cyclodextrin
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical ...
-based polymer to form the investigational anti-cancer drug CRLX101 CRLX101 is an experimental approach to cancer chemotherapy that is under investigation in human trials. It is an example of a nanomedicine.
The agent represents a nanoparticle conjugate that consists of a drug delivery molecule, namely a cyclodext ...
.
Biosynthesis


 Like all other monoterpenoid indole-alkaloids, biosynthesis of camptothecin requires production of the
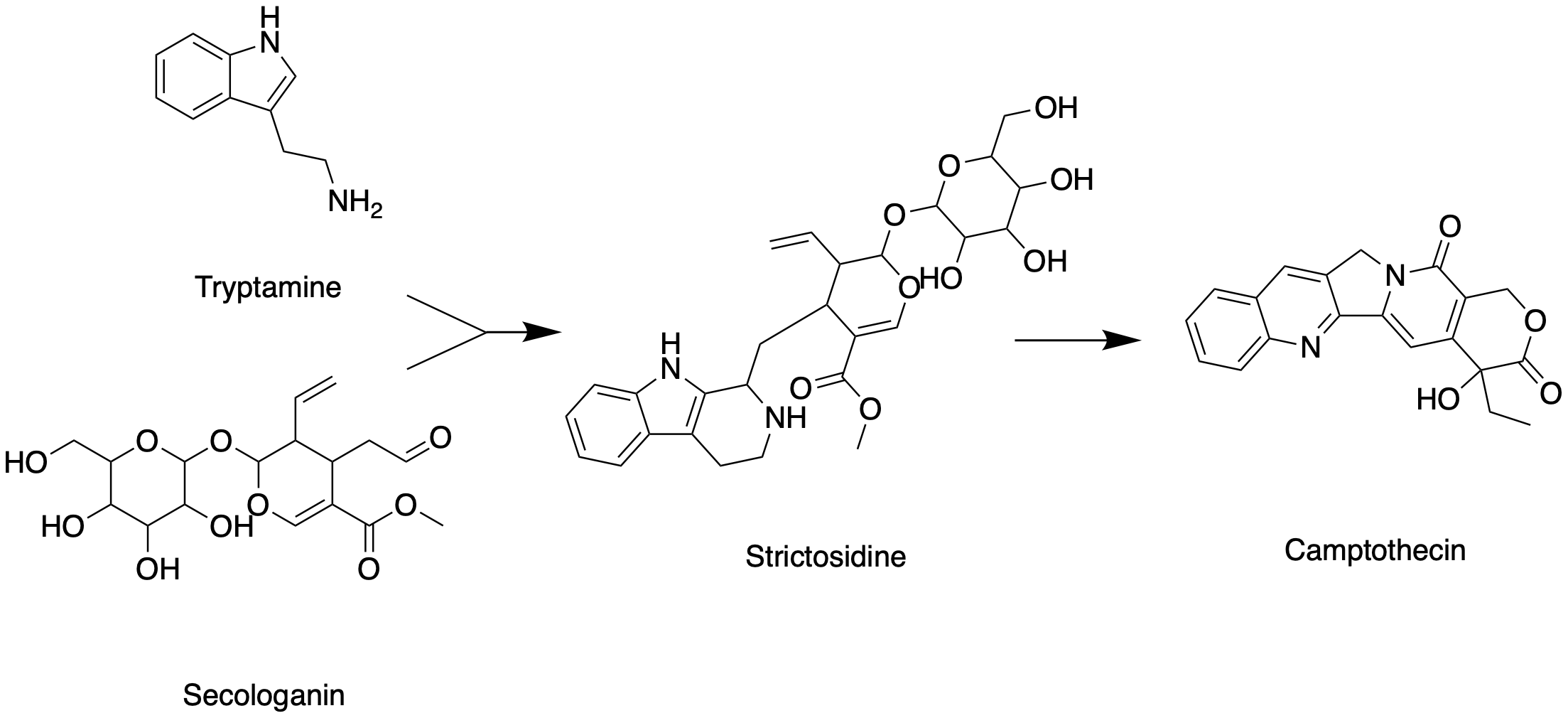
Like all other monoterpenoid indole-alkaloids, biosynthesis of camptothecin requires production of the strictosidine
Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. ...
. Strictosidine is synthesized through condensation reaction between trypamine from shikimate pathway and secologanin from either mevalonate (MVA) pathway or non-mevalonate pathway (MEP). Strictosidine then undergoes intermolecular cyclization to produce strictosamide, which is converted to camptothecin through a series of oxidation reactions by enzymes that still needs to be resolved.
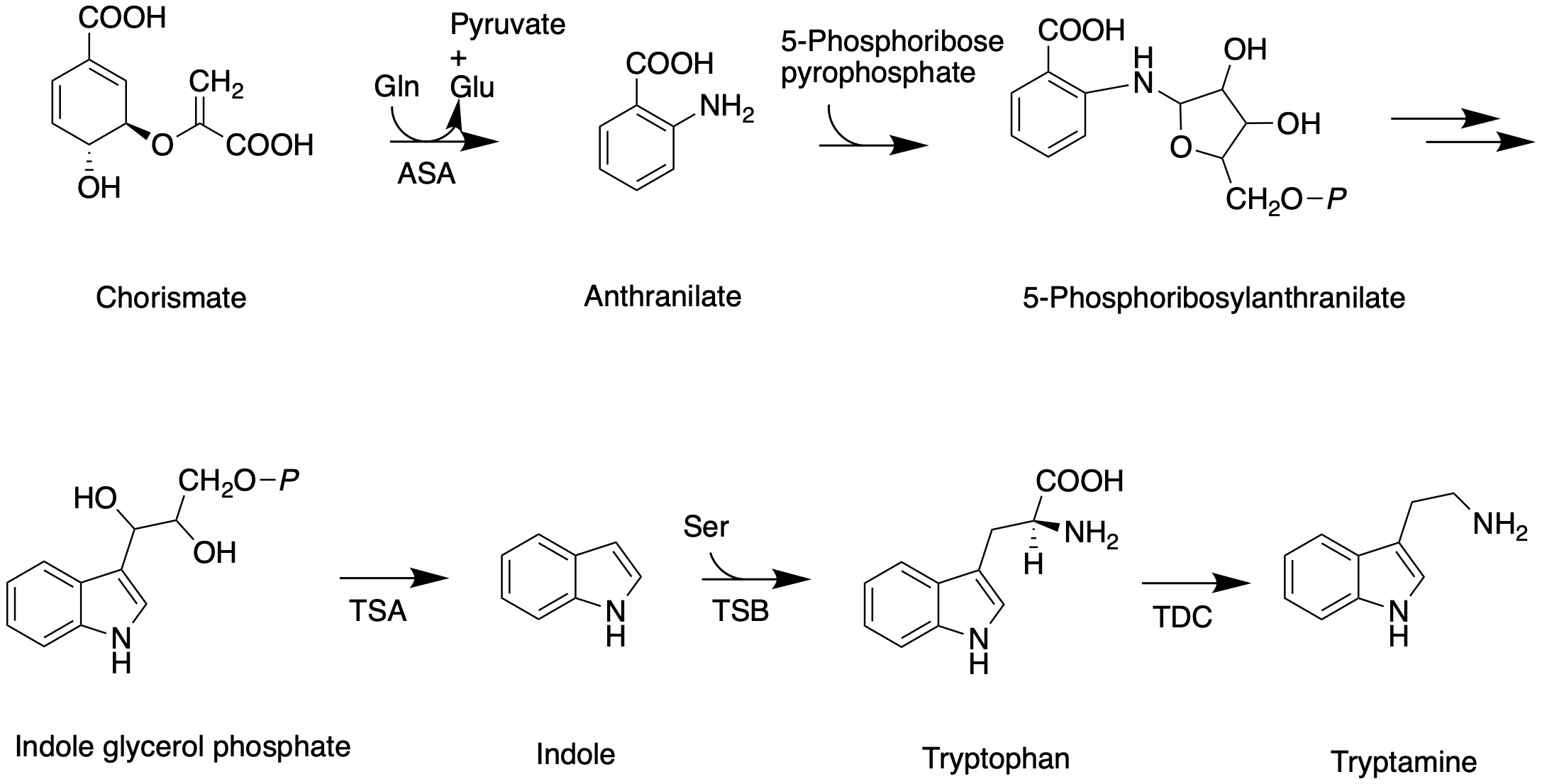
The shikimate pathway leading to biosynthesis of tryptamine is mostly understood. First, chorismate is converted to anthranilate by the alpha-subunit of anthranilate synthase (ASA). Anthranilate reacts with 5-phosphoribose pyrrophosphate to produce 5-phosphoribosylanthranilate. Then this intermediate is converted to indole glycerol phosphate, which interacts with the alpha-subunit of tryptophan (TSA) synthase to yield indole. The beta-subunit of tryptophan synthase (TSB) catalyzes condensation of indole with serine, leading to tryptophan. In the next step, tryptoamine is produced as the result of decarboxylation by tryptophan decarboxylase (TDC).
Secologanin synthesis begins with condensation reaction between pyruvate and D-Glyceraldehyde-3-phosphate catalyzed by 1-deoxy-D-xylulose-5-phosphate synthase (DXS) to produce 1-deoxy-D-xylulose-5-phosphate (DXP). The conversion of DXP to isopentenyl diphosphate (IPP), which is the common terpenoid biosynthesis precursor involves 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR) and 1-hydroxy-2-methyl-2(E)-butenyl-4-diphosphate reductase (HDR).
The formation of IPP can be achieved by both MVA and MEP pathways. Condensation of IPP and dimethylallyl diphosphate (DMAPP) yields geranyl diphosphate (GPP). The geraniol synthase (GS) then converts GPP to geraniol. The conversion of geraniol to secologanin occurs through various enzymatic reactions. Based on studies with radioactive labelling and pathway specific inhibitors, MEP pathway is the primary source for secologanin.
Tryptamine from shikimate pathway and secologanin from MVA or MEP pathway are converted to strictosidine through a condensation reaction catalyzed by strictosidine synthase. Although it is not fully resolved, it has been postulated that camptothecin is produced from strictosidine via strictosamide, 3 (S)-pumiloside and 3 (S)-deoxypumiloside.
References
{{Chemotherapeutic agents Topoisomerase inhibitors Quinoline alkaloids Tertiary alcohols Delta-lactones Delta-lactams Pyranoindolizinoquinolines Enones Conjugated dienes Plant toxins