Closo on:
[Wikipedia]
[Google]
[Amazon]
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as
 Different rules (4''n'', 5''n'', or 6''n'') are invoked depending on the number of electrons per vertex.
The 4''n'' rules are reasonably accurate in predicting the structures of clusters having about 4 electrons per vertex, as is the case for many
Different rules (4''n'', 5''n'', or 6''n'') are invoked depending on the number of electrons per vertex.
The 4''n'' rules are reasonably accurate in predicting the structures of clusters having about 4 electrons per vertex, as is the case for many
 The following
The following  When counting electrons for each cluster, the number of
When counting electrons for each cluster, the number of  Other rules may be considered when predicting the structure of clusters:
# For clusters consisting mostly of transition metals, any main group elements present are often best counted as ligands or interstitial atoms, rather than vertices.
# Larger and more electropositive atoms tend to occupy vertices of high connectivity and smaller more electronegative atoms tend to occupy vertices of low connectivity.
# In the special case of boron hydride clusters, each boron atom connected to 3 or more vertices has one terminal hydride, while a boron atom connected to two other vertices has two terminal hydrogen atoms. If more hydrogen atoms are present, they are placed in open face positions to even out the coordination number of the vertices.
# For the special case of transition metal clusters, ligands are added to the metal centers to give the metals reasonable coordination numbers, and if any
Other rules may be considered when predicting the structure of clusters:
# For clusters consisting mostly of transition metals, any main group elements present are often best counted as ligands or interstitial atoms, rather than vertices.
# Larger and more electropositive atoms tend to occupy vertices of high connectivity and smaller more electronegative atoms tend to occupy vertices of low connectivity.
# In the special case of boron hydride clusters, each boron atom connected to 3 or more vertices has one terminal hydride, while a boron atom connected to two other vertices has two terminal hydrogen atoms. If more hydrogen atoms are present, they are placed in open face positions to even out the coordination number of the vertices.
# For the special case of transition metal clusters, ligands are added to the metal centers to give the metals reasonable coordination numbers, and if any  Example:
:Electron count: 10 × Pb + 2 (for the negative charge) = 10 × 4 + 2 = 42 electrons.
:Since ''n'' = 10, 4''n'' + 2 = 42, so the cluster is a ''closo'' bicapped square antiprism.
Example:
:Electron count: 4 × S – 2 (for the positive charge) = 4 × 6 – 2 = 22 electrons.
:Since ''n'' = 4, 4''n'' + 6 = 22, so the cluster is ''arachno''.
:Starting from an octahedron, a vertex of high connectivity is removed, and then a non-adjacent vertex is removed.
Example: Os6(CO)18
:Electron count: 6 × Os + 18 × CO – 60 (for 6 osmium atoms) = 6 × 8 + 18 × 2 – 60 = 24
:Since ''n'' = 6, 4''n'' = 24, so the cluster is capped ''closo''.
:Starting from a trigonal bipyramid, a face is capped. The carbonyls have been omitted for clarity.
Example:
:Electron count: 10 × Pb + 2 (for the negative charge) = 10 × 4 + 2 = 42 electrons.
:Since ''n'' = 10, 4''n'' + 2 = 42, so the cluster is a ''closo'' bicapped square antiprism.
Example:
:Electron count: 4 × S – 2 (for the positive charge) = 4 × 6 – 2 = 22 electrons.
:Since ''n'' = 4, 4''n'' + 6 = 22, so the cluster is ''arachno''.
:Starting from an octahedron, a vertex of high connectivity is removed, and then a non-adjacent vertex is removed.
Example: Os6(CO)18
:Electron count: 6 × Os + 18 × CO – 60 (for 6 osmium atoms) = 6 × 8 + 18 × 2 – 60 = 24
:Since ''n'' = 6, 4''n'' = 24, so the cluster is capped ''closo''.
:Starting from a trigonal bipyramid, a face is capped. The carbonyls have been omitted for clarity.
 Example:
:Electron count: 5 × B + 5 × H + 4 (for the negative charge) = 5 × 3 + 5 × 1 + 4 = 24
:Since ''n'' = 5, 4''n'' + 4 = 24, so the cluster is nido.
:Starting from an octahedron, one of the vertices is removed.
The rules are useful in also predicting the structure of
Example:
:Electron count: 5 × B + 5 × H + 4 (for the negative charge) = 5 × 3 + 5 × 1 + 4 = 24
:Since ''n'' = 5, 4''n'' + 4 = 24, so the cluster is nido.
:Starting from an octahedron, one of the vertices is removed.
The rules are useful in also predicting the structure of
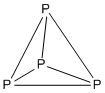

 The 5''n'' rules are as follows.
Example: P4
:Electron count: 4 × P = 4 × 5 = 20
:It is a 5''n'' structure with ''n'' = 4, so it is tetrahedral
Example: P4S3
:Electron count 4 × P + 3 × S = 4 × 5 + 3 × 6 = 38
:It is a 5''n'' + 3 structure with ''n'' = 7. Three vertices are inserted into edges
Example: P4O6
:Electron count 4 × P + 6 × O = 4 × 5 + 6 × 6 = 56
:It is a 5''n'' + 6 structure with ''n'' = 10. Six vertices are inserted into edges
The 5''n'' rules are as follows.
Example: P4
:Electron count: 4 × P = 4 × 5 = 20
:It is a 5''n'' structure with ''n'' = 4, so it is tetrahedral
Example: P4S3
:Electron count 4 × P + 3 × S = 4 × 5 + 3 × 6 = 38
:It is a 5''n'' + 3 structure with ''n'' = 7. Three vertices are inserted into edges
Example: P4O6
:Electron count 4 × P + 6 × O = 4 × 5 + 6 × 6 = 56
:It is a 5''n'' + 6 structure with ''n'' = 10. Six vertices are inserted into edges
 Example: S8
:Electron count = 8 × S = 8 × 6 = 48 electrons.
:Since ''n'' = 8, 6''n'' = 48, so the cluster is an 8-membered ring.
Example: S8
:Electron count = 8 × S = 8 × 6 = 48 electrons.
:Since ''n'' = 8, 6''n'' = 48, so the cluster is an 8-membered ring.
 Hexane (C6H14)
:Electron count = 6 × C + 14 × H = 6 × 4 + 14 × 1 = 38
:Since ''n'' = 6, 6''n'' = 36 and 6''n'' + 2 = 38, so the cluster is a 6-membered chain.
Hexane (C6H14)
:Electron count = 6 × C + 14 × H = 6 × 4 + 14 × 1 = 38
:Since ''n'' = 6, 6''n'' = 36 and 6''n'' + 2 = 38, so the cluster is a 6-membered chain.
 :The boron atoms lie on each vertex of the octahedron and are sp hybridized. One sp-hybrid radiates away from the structure forming the bond with the hydrogen atom. The other sp-hybrid radiates into the center of the structure forming a large bonding molecular orbital at the center of the cluster. The remaining two unhybridized orbitals lie along the tangent of the sphere like structure creating more bonding and antibonding orbitals between the boron vertices. The orbital diagram breaks down as follows:
::The 18 framework molecular orbitals, (MOs), derived from the 18 boron atomic orbitals are:
::*1 bonding MO at the center of the cluster and 5 antibonding MOs from the 6 sp-radial hybrid orbitals
::*6 bonding MOs and 6 antibonding MOs from the 12 tangential p-orbitals.
:The total skeletal bonding orbitals is therefore 7, i.e. .
:The boron atoms lie on each vertex of the octahedron and are sp hybridized. One sp-hybrid radiates away from the structure forming the bond with the hydrogen atom. The other sp-hybrid radiates into the center of the structure forming a large bonding molecular orbital at the center of the cluster. The remaining two unhybridized orbitals lie along the tangent of the sphere like structure creating more bonding and antibonding orbitals between the boron vertices. The orbital diagram breaks down as follows:
::The 18 framework molecular orbitals, (MOs), derived from the 18 boron atomic orbitals are:
::*1 bonding MO at the center of the cluster and 5 antibonding MOs from the 6 sp-radial hybrid orbitals
::*6 bonding MOs and 6 antibonding MOs from the 12 tangential p-orbitals.
:The total skeletal bonding orbitals is therefore 7, i.e. .
borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated ...
and carborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these c ...
clusters. The electron counting rules were originally formulated by Kenneth Wade
Kenneth Wade, (1932–2014) was a British chemist and professor emeritus at Durham University.
Early life and education
Kenneth Wade was born in Sleaford on 13 October 1932, the second son of Harry Kennington Wade and his wife, Anna Elizabet ...
, and were further developed by others including Michael Mingos
David Michael Patrick Mingos, FRS (born 6 August 1944) is a British chemist and academic. He was Principal of St Edmund Hall, Oxford from 1999 to 2009, and Professor of Inorganic Chemistry at the University of Oxford.
Education
Mingos attended ...
; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
treatment of the bonding. These notes contained original material that served as the basis of the sections on the 4''n'', 5''n'', and 6''n'' rules. These rules have been extended and unified in the form of the Jemmis ''mno'' rules.
Predicting structures of cluster compounds
boranes
Boranes is the name given to compounds with the formula BxHy and related anions. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bond ...
and carborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these c ...
s. For such clusters, the structures are based on deltahedra
In geometry, a deltahedron (plural ''deltahedra'') is a polyhedron whose faces are all equilateral triangles. The name is taken from the Greek upper case delta (Δ), which has the shape of an equilateral triangle. There are infinitely many del ...
, which are polyhedra
In geometry, a polyhedron (plural polyhedra or polyhedrons; ) is a three-dimensional shape with flat polygonal faces, straight edges and sharp corners or vertices.
A convex polyhedron is the convex hull of finitely many points, not all on ...
in which every face is triangular. The 4''n'' clusters are classified as ''closo-'', ''nido-'', ''arachno-'' or ''hypho-'', based on whether they represent a complete (''closo-'') deltahedron
In geometry, a deltahedron (plural ''deltahedra'') is a polyhedron whose faces are all equilateral triangles. The name is taken from the Greek upper case delta (Δ), which has the shape of an equilateral triangle. There are infinitely many d ...
, or a deltahedron that is missing one (''nido-''), two (''arachno-'') or three (''hypho-'') vertices.
However, hypho clusters are relatively uncommon due to the fact that the electron count is high enough to start to fill antibonding orbitals and destabilize the 4''n'' structure. If the electron count is close to 5 electrons per vertex, the structure often changes to one governed by the 5n rules, which are based on 3-connected polyhedra.
As the electron count increases further, the structures of clusters with 5n electron counts become unstable, so the 6''n'' rules can be implemented. The 6''n'' clusters have structures that are based on rings.
A molecular orbital treatment can be used to rationalize the bonding of cluster compounds of the 4''n'', 5''n'', and 6''n'' types.
4''n'' rules
 The following
The following polyhedra
In geometry, a polyhedron (plural polyhedra or polyhedrons; ) is a three-dimensional shape with flat polygonal faces, straight edges and sharp corners or vertices.
A convex polyhedron is the convex hull of finitely many points, not all on ...
are ''closo'' polyhedra, and are the basis for the 4''n'' rules; each of these have triangular faces. The number of vertices in the cluster determines what polyhedron the structure is based on.
Using the electron count, the predicted structure can be found. ''n'' is the number of vertices in the cluster. The 4''n'' rules are enumerated in the following table.
 When counting electrons for each cluster, the number of
When counting electrons for each cluster, the number of valence electrons
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
is enumerated. For each transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
present, 10 electrons are subtracted from the total electron count. For example, in Rh6(CO)16 the total number of electrons would be = = 26. Therefore, the cluster is a ''closo'' polyhedron because , with .
 Other rules may be considered when predicting the structure of clusters:
# For clusters consisting mostly of transition metals, any main group elements present are often best counted as ligands or interstitial atoms, rather than vertices.
# Larger and more electropositive atoms tend to occupy vertices of high connectivity and smaller more electronegative atoms tend to occupy vertices of low connectivity.
# In the special case of boron hydride clusters, each boron atom connected to 3 or more vertices has one terminal hydride, while a boron atom connected to two other vertices has two terminal hydrogen atoms. If more hydrogen atoms are present, they are placed in open face positions to even out the coordination number of the vertices.
# For the special case of transition metal clusters, ligands are added to the metal centers to give the metals reasonable coordination numbers, and if any
Other rules may be considered when predicting the structure of clusters:
# For clusters consisting mostly of transition metals, any main group elements present are often best counted as ligands or interstitial atoms, rather than vertices.
# Larger and more electropositive atoms tend to occupy vertices of high connectivity and smaller more electronegative atoms tend to occupy vertices of low connectivity.
# In the special case of boron hydride clusters, each boron atom connected to 3 or more vertices has one terminal hydride, while a boron atom connected to two other vertices has two terminal hydrogen atoms. If more hydrogen atoms are present, they are placed in open face positions to even out the coordination number of the vertices.
# For the special case of transition metal clusters, ligands are added to the metal centers to give the metals reasonable coordination numbers, and if any hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atoms are present they are placed in bridging positions to even out the coordination numbers of the vertices.
In general, ''closo'' structures with ''n'' vertices are ''n''-vertex polyhedra.
To predict the structure of a ''nido'' cluster, the ''closo'' cluster with ''n'' + 1 vertices is used as a starting point; if the cluster is composed of small atoms a high connectivity vertex is removed, while if the cluster is composed of large atoms a low connectivity vertex is removed.
To predict the structure of an ''arachno'' cluster, the ''closo'' polyhedron with ''n'' + 2 vertices is used as the starting point, and the ''n'' + 1 vertex ''nido'' complex is generated by following the rule above; a second vertex adjacent to the first is removed if the cluster is composed of mostly small atoms, a second vertex not adjacent to the first is removed if the cluster is composed mostly of large atoms.
 Example:
:Electron count: 10 × Pb + 2 (for the negative charge) = 10 × 4 + 2 = 42 electrons.
:Since ''n'' = 10, 4''n'' + 2 = 42, so the cluster is a ''closo'' bicapped square antiprism.
Example:
:Electron count: 4 × S – 2 (for the positive charge) = 4 × 6 – 2 = 22 electrons.
:Since ''n'' = 4, 4''n'' + 6 = 22, so the cluster is ''arachno''.
:Starting from an octahedron, a vertex of high connectivity is removed, and then a non-adjacent vertex is removed.
Example: Os6(CO)18
:Electron count: 6 × Os + 18 × CO – 60 (for 6 osmium atoms) = 6 × 8 + 18 × 2 – 60 = 24
:Since ''n'' = 6, 4''n'' = 24, so the cluster is capped ''closo''.
:Starting from a trigonal bipyramid, a face is capped. The carbonyls have been omitted for clarity.
Example:
:Electron count: 10 × Pb + 2 (for the negative charge) = 10 × 4 + 2 = 42 electrons.
:Since ''n'' = 10, 4''n'' + 2 = 42, so the cluster is a ''closo'' bicapped square antiprism.
Example:
:Electron count: 4 × S – 2 (for the positive charge) = 4 × 6 – 2 = 22 electrons.
:Since ''n'' = 4, 4''n'' + 6 = 22, so the cluster is ''arachno''.
:Starting from an octahedron, a vertex of high connectivity is removed, and then a non-adjacent vertex is removed.
Example: Os6(CO)18
:Electron count: 6 × Os + 18 × CO – 60 (for 6 osmium atoms) = 6 × 8 + 18 × 2 – 60 = 24
:Since ''n'' = 6, 4''n'' = 24, so the cluster is capped ''closo''.
:Starting from a trigonal bipyramid, a face is capped. The carbonyls have been omitted for clarity.
 Example:
:Electron count: 5 × B + 5 × H + 4 (for the negative charge) = 5 × 3 + 5 × 1 + 4 = 24
:Since ''n'' = 5, 4''n'' + 4 = 24, so the cluster is nido.
:Starting from an octahedron, one of the vertices is removed.
The rules are useful in also predicting the structure of
Example:
:Electron count: 5 × B + 5 × H + 4 (for the negative charge) = 5 × 3 + 5 × 1 + 4 = 24
:Since ''n'' = 5, 4''n'' + 4 = 24, so the cluster is nido.
:Starting from an octahedron, one of the vertices is removed.
The rules are useful in also predicting the structure of carborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these c ...
s.
Example: C2B7H13
:Electron count = 2 × C + 7 × B + 13 × H = 2 × 4 + 7 × 3 + 13 × 1 = 42
:Since n in this case is 9, 4''n'' + 6 = 42, the cluster is ''arachno''.
The bookkeeping for deltahedral clusters is sometimes carried out by counting skeletal electrons instead of the total number of electrons. The skeletal orbital (electron pair) and skeletal electron counts for the four types of deltahedral clusters are:
*''n''-vertex ''closo'': ''n'' + 1 skeletal orbitals, 2''n'' + 2 skeletal electrons
*''n''-vertex ''nido'': ''n'' + 2 skeletal orbitals, 2''n'' + 4 skeletal electrons
*''n''-vertex ''arachno'': ''n'' + 3 skeletal orbitals, 2''n'' + 6 skeletal electrons
*''n''-vertex ''hypho'': ''n'' + 4 skeletal orbitals, 2''n'' + 8 skeletal electrons
The skeletal electron counts are determined by summing the total of the following number of electrons:
*2 from each BH unit
*3 from each CH unit
*1 from each additional hydrogen atom (over and above the ones on the BH and CH units)
*the anionic charge electrons
5''n'' rules
As discussed previously, the 4''n'' rule mainly deals with clusters with electron counts of , in which approximately 4electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
are on each vertex. As more electrons are added per vertex, the number of the electrons per vertex approaches 5. Rather than adopting structures based on deltahedra, the 5n-type clusters have structures based on a different series of polyhedra known as the 3-connected polyhedra
In geometry, a polyhedron (plural polyhedra or polyhedrons; ) is a three-dimensional shape with flat polygonal faces, straight edges and sharp corners or vertices.
A convex polyhedron is the convex hull of finitely many points, not all on ...
, in which each vertex is connected to 3 other vertices. The 3-connected polyhedra are the duals
''Duals'' is a compilation album by the Irish rock band U2. It was released in April 2011 to u2.com subscribers.
Track listing
:* "Where the Streets Have No Name" and "Amazing Grace" are studio mix of U2's performance at the Rose Bowl, P ...
of the deltahedra. The common types of 3-connected polyhedra are listed below.


6''n'' rules
As more electrons are added to a 5''n'' cluster, the number of electrons per vertex approaches 6. Instead of adopting structures based on 4''n'' or 5''n'' rules, the clusters tend to have structures governed by the 6''n'' rules, which are based on rings. The rules for the 6''n'' structures are as follows.Isolobal vertex units
Provided a vertex unit isisolobal
In organometallic chemistry, the isolobal principle (more formally known as the isolobal analogy) is a strategy used to relate the structure of organic and inorganic molecular fragments in order to predict bonding properties of organometallic comp ...
with BH then it can, in principle at least, be substituted for a BH unit, even though BH and CH are not isoelectronic. The CH+ unit is isolobal, hence the rules are applicable to carboranes. This can be explained due to a frontier orbital
A frontier is the political and geographical area near or beyond a boundary. A frontier can also be referred to as a "front". The term came from French in the 15th century, with the meaning "borderland"—the region of a country that fronts o ...
treatment. Additionally there are isolobal transition-metal units. For example, Fe(CO)3 provides 2 electrons. The derivation of this is briefly as follows:
*Fe has 8 valence electrons.
*Each carbonyl group is a net 2 electron donor after the internal σ- and π-bonding are taken into account making 14 electrons.
*3 pairs are considered to be involved in Fe–CO σ-bonding and 3 pairs are involved in π-backbonding from Fe to CO reducing the 14 to 2.
Bonding in cluster compounds
;''closo''- :The boron atoms lie on each vertex of the octahedron and are sp hybridized. One sp-hybrid radiates away from the structure forming the bond with the hydrogen atom. The other sp-hybrid radiates into the center of the structure forming a large bonding molecular orbital at the center of the cluster. The remaining two unhybridized orbitals lie along the tangent of the sphere like structure creating more bonding and antibonding orbitals between the boron vertices. The orbital diagram breaks down as follows:
::The 18 framework molecular orbitals, (MOs), derived from the 18 boron atomic orbitals are:
::*1 bonding MO at the center of the cluster and 5 antibonding MOs from the 6 sp-radial hybrid orbitals
::*6 bonding MOs and 6 antibonding MOs from the 12 tangential p-orbitals.
:The total skeletal bonding orbitals is therefore 7, i.e. .
:The boron atoms lie on each vertex of the octahedron and are sp hybridized. One sp-hybrid radiates away from the structure forming the bond with the hydrogen atom. The other sp-hybrid radiates into the center of the structure forming a large bonding molecular orbital at the center of the cluster. The remaining two unhybridized orbitals lie along the tangent of the sphere like structure creating more bonding and antibonding orbitals between the boron vertices. The orbital diagram breaks down as follows:
::The 18 framework molecular orbitals, (MOs), derived from the 18 boron atomic orbitals are:
::*1 bonding MO at the center of the cluster and 5 antibonding MOs from the 6 sp-radial hybrid orbitals
::*6 bonding MOs and 6 antibonding MOs from the 12 tangential p-orbitals.
:The total skeletal bonding orbitals is therefore 7, i.e. .
Transition metal clusters
Transition metal clusters use the d orbitals for bonding. Thus they have up to nine bonding orbitals, instead of only the four present in boron and main group clusters.Clusters with interstitial atoms
Owing their large radii, transition metals generally form clusters that are larger than main group elements. One consequence of their increased size, these clusters often contain atoms at their centers. A prominent example is e6C(CO)16sup>2-. In such cases, the rules of electron counting assume that the interstitial atom contributes all valence electrons to cluster bonding. In this way, e6C(CO)16sup>2- is equivalent to e6(CO)16sup>6- or e6(CO)18sup>2-.References
General references
* * {{DEFAULTSORT:Polyhedral Skeletal Electron Pair Theory Chemical bonding Inorganic chemistry Organometallic chemistry Cluster chemistry