Claus Process on:
[Wikipedia]
[Google]
[Amazon]
 The Claus process is the most significant gas desulfurizing process, recovering elemental
The Claus process is the most significant gas desulfurizing process, recovering elemental
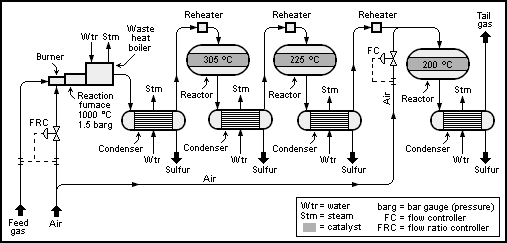 The Claus technology can be divided into two process steps, thermal and
The Claus technology can be divided into two process steps, thermal and
 The Claus process is the most significant gas desulfurizing process, recovering elemental
The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus
Carl Friedrich Claus (born 9 November 1827 in Kassel; died 29 August 1900 in London) was a German chemist and inventor. He patented the Claus process.
Life
Claus studied chemistry at University of Marburg in Germany. He emigrated to England, ...
, the Claus process has become the industry standard.
The multi-step Claus process recovers sulfur from the gaseous hydrogen sulfide found in raw natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
and from the by-product gases containing hydrogen sulfide derived from refining crude oil
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crude ...
and other industrial processes. The by-product gases mainly originate from physical and chemical gas treatment units (Selexol
Selexol is the trade name for an acid gas removal solvent that can separate acid gases such as hydrogen sulfide and carbon dioxide from feed gas streams such as synthesis gas produced by gasification of coal, coke, or heavy hydrocarbon oils. By ...
, Rectisol Rectisol is the trade name for an acid gas removal process that uses methanol as a solvent to separate acid gases such as hydrogen sulfide and carbon dioxide from valuable feed gas streams. By doing so, the feed gas is made more suitable for combus ...
, Purisol and amine scrubbers) in refineries, natural gas processing plants and gasification or synthesis gas plants. These by-product gases may also contain hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
, hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
or ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
.
Gases with an H2S content of over 25% are suitable for the recovery of sulfur in straight-through Claus plants while alternate configurations such as a split-flow set up or feed and air preheating can be used to process leaner feeds.
Hydrogen sulfide produced, for example, in the hydro-desulfurization of refinery naphtha
Naphtha ( or ) is a flammable liquid hydrocarbon mixture.
Mixtures labelled ''naphtha'' have been produced from natural gas condensates, petroleum distillates, and the distillation of coal tar and peat. In different industries and regions ' ...
s and other petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
oils, is converted to sulfur in Claus plants. The reaction proceeds in two steps:
:2 H2S +3 O2 → 2 SO2 + 2 H2O
:4 H2S +2 SO2 → 3 S2 + 4 H2O
The vast majority of the 64,000,000 tonnes of sulfur produced worldwide in 2005 was byproduct sulfur from refineries and other hydrocarbon processing plants.''Der Claus-Prozess. Reich an Jahren und bedeutender denn je'', Bernhard Schreiner, Chemie in Unserer Zeit 2008, Vol. 42, Issue 6, Pages 378-392. Sulfur is used for manufacturing sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
, medicine, cosmetics, fertilizers and rubber products. Elemental sulfur is used as fertilizer and pesticide.
History
The process was invented byCarl Friedrich Claus
Carl Friedrich Claus (born 9 November 1827 in Kassel; died 29 August 1900 in London) was a German chemist and inventor. He patented the Claus process.
Life
Claus studied chemistry at University of Marburg in Germany. He emigrated to England, ...
, a German chemist working in England. A British patent was issued to him in 1883. The process was later significantly modified by IG Farben.
Claus was born in Kassel
Kassel (; in Germany, spelled Cassel until 1926) is a city on the Fulda River in northern Hesse, Germany. It is the administrative seat of the Regierungsbezirk Kassel and the district of the same name and had 201,048 inhabitants in December 2020 ...
in the German State of Hesse in 1827, and studied chemistry in Marburg
Marburg ( or ) is a university town in the German federal state (''Bundesland'') of Hesse, capital of the Marburg-Biedenkopf district (''Landkreis''). The town area spreads along the valley of the river Lahn and has a population of approxima ...
before he emigrated to England in 1852. He died in London in 1900.
Process description
A schematic process flow diagram of a basic 2+1-reactor (converter) SuperClaus unit is shown below: The Claus technology can be divided into two process steps, thermal and
The Claus technology can be divided into two process steps, thermal and catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
.
Thermal step
In the thermal step, hydrogen sulfide-laden gas reacts in a substoichiometriccombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
at temperatures above 850 °C Or between 950 and 1200 °C and even hotter near the flame, as stated in ''Der Claus-Prozess. Reich an Jahren und bedeutender denn je'', Bernhard Schreiner, Chemie in Unserer Zeit 2008, Vol. 42, Issue 6, Pages 378-392. such that elemental sulfur precipitates in the downstream process gas cooler.
The H2S content and the concentration of other combustible components (hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s or ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
) determine the location where the feed gas is burned. Claus gases (acid gas) with no further combustible contents apart from H2S are burned in lances surrounding a central muffle by the following chemical reaction:
:2 H2S + 3 O2 → 2 SO2 + 2 H2O (Δ''H'' = -518 kJ mol−1)
This is a strongly exothermic free-flame total oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of hydrogen sulfide generating sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
that reacts away in subsequent reactions. The most important one is the Claus reaction:
:2 H2S + SO2 → 3 S + 2 H2O
The overall equation is:
:2 H2S + O2 → 2 S + 2 H2O
The temperature inside Claus furnace is often maintained above 1050°C. This ensures BTEX (Benzene, Toluene, Ethyl benzene and Xylene) destruction which otherwise would clog downstream Claus catalyst.
Gases containing ammonia, such as the gas from the refinery's sour water stripper (SWS), or hydrocarbons are converted in the burner muffle. Sufficient air is injected into the muffle for the complete combustion of all hydrocarbons and ammonia. The air to the acid gas ratio is controlled such that in total 1/3 of all hydrogen sulfide (H2S) is converted to SO2. This ensures a stoichiometric reaction for the Claus reaction in the second catalytic step (see next section below).
The separation of the combustion processes ensures an accurate dosage of the required air volume needed as a function of the feed gas composition. To reduce the process gas volume or obtain higher combustion temperatures, the air requirement can also be covered by injecting pure oxygen. Several technologies utilizing high-level and low-level oxygen enrichment are available in industry, which requires the use of a special burner in the reaction furnace for this process option.
Usually, 60 to 70% of the total amount of elemental sulfur produced in the process is obtained in the thermal process step.
The main portion of the hot gas from the combustion chamber flows through the tube of the process gas cooler and is cooled down such that the sulfur formed in the reaction step condenses
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
. The heat given off by the process gas and the condensation heat evolved are utilized to produce medium or low-pressure steam. The condensed sulfur is removed at the liquid outlet section of the process gas cooler.
The sulfur forms in the thermal phase as highly reactive S2 diradicals which combine exclusively to the S8 allotrope:
: 4 S2 → S8
Side reactions
Other chemical processes taking place in the thermal step of the Claus reaction are: * The formation ofhydrogen gas
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, a ...
:
:2 H2S → S2 + 2 H2 (Δ''H'' > 0)
: CH4 + 2 H2O → CO2 + 4 H2
* The formation of carbonyl sulfide
Carbonyl sulfide is the chemical compound with the linear formula OCS. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom. Carbonyl sulfide can be consi ...
:
: H2S + CO2 → S=C=O + H2O
* The formation of carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
:
: CH4 + 2 S2 → S=C=S + 2 H2S
Catalytic step
The Claus reaction continues in thecatalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
step with activated aluminum(III) or titanium(IV) oxide, and serves to boost the sulfur yield. More hydrogen sulfide ( H2S) reacts with the SO2 formed during combustion in the reaction furnace in the Claus reaction, and results in gaseous, elemental sulfur.
:2 H2S + SO2 → 3 S + 2 H2O (Δ''H'' = -1165.6 kJ mol−1)
One suggested mechanism is that S6 and S8 desorb from the catalyst's active sites with simultaneous formation of stable cyclic elemental sulfur.
The catalytic recovery of sulfur consists of three substeps: heating, catalytic reaction and cooling plus condensation.
These three steps are normally repeated a maximum of three times. Where an incineration or tail-gas treatment unit (TGTU) is added downstream of the Claus plant, only two catalytic stages are usually installed.
The first process step in the catalytic stage is the gas heating process. It is necessary to prevent sulfur condensation in the catalyst bed, which can lead to catalyst fouling. The required bed operating temperature in the individual catalytic stages is achieved by heating the process gas in a reheater until the desired operating bed temperature is reached.
Several methods of reheating are used in industry:
* Hot-gas bypass: which involves mixing the two process gas streams from the process gas cooler (cold gas) and the bypass (hot gas) from the first pass of the waste-heat boiler.
* Indirect steam reheaters: the gas can also be heated with high-pressure steam in a heat exchanger.
* Gas/gas exchangers: whereby the cooled gas from the process gas cooler is indirectly heated from the hot gas coming out of an upstream catalytic reactor in a gas-to-gas exchanger.
* Direct-fired heaters: fired reheaters utilizing acid gas or fuel gas, which is burned substoichiometrically to avoid oxygen breakthrough which can damage Claus catalyst.
The typically recommended operating temperature of the first catalyst stage is 315 °C to 330 °C (bottom bed temperature). The high temperature in the first stage also helps to hydrolyze COS and CS2, which is formed in the furnace and would not otherwise be converted in the modified Claus process.
The catalytic conversion is maximized at lower temperatures, but care must be taken to ensure that each bed is operated above the dew point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will cond ...
of sulfur. The operating temperatures of the subsequent catalytic stages are typically 240 °C for the second stage and 200 °C for the third stage (bottom bed temperatures).
In the sulfur condenser, the process gas coming from the catalytic reactor is cooled to between 150 and 130 °C. The condensation heat is used to generate steam at the shell side of the condenser.
Before storage, liquid sulfur streams from the process gas cooler, the sulfur condensers and from the final sulfur separator are routed to the degassing unit, where the gases (primarily H2S) dissolved in the sulfur are removed.
The tail gas from the Claus process still containing combustible components and sulfur compounds (H2S, H2 and CO) is either burned in an incineration unit or further desulfurized in a downstream tail gas treatment unit.
Sub dew point Claus process
The conventional Claus process described above is limited in its conversion due to the reaction equilibrium being reached. Like all exothermic reactions, greater conversion can be achieved at lower temperatures, however as mentioned the Claus reactor must be operated above the sulfur dew point (120–150 °C) to avoid liquid sulfur physically deactivating the catalyst. To overcome this problem, the sub dew point Clauss reactors are oriented in parallel, with one operating and one spare. When one reactor has become saturated with adsorbed sulfur, the process flow is diverted to the standby reactor. The reactor is then regenerated by sending process gas that has been heated to 300–350 °C to vaporize the sulfur. This stream is sent to a condenser to recover the sulfur.Process performance
Over 2.6 tons of steam will be generated for each ton of sulfur yield. The physical properties of elemental sulfur obtained in the Claus process can differ from that obtained by other processes. Sulfur is usually transported as a liquid (melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
115 °C). In elemental sulfur, viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
increases rapidly at temperatures in excess of 160 °C due to the formation of polymeric sulfur chains. Another anomaly is found in the solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
of residual H2S in liquid sulfur as a function of temperature. Ordinarily, the solubility of a gas increases with increasing temperature but with H2S it is the opposite. This means that toxic and explosive H2S gas can build up in the headspace of any cooling liquid sulfur reservoir. The explanation for this anomaly is the endothermic reaction of sulfur with H2S to polysulfanes H2Sx.
Sulfur stockpile
Huge amounts of elemental sulfur (billions of tons) are produced worldwide by the Claus process. The process has also to be applied to heavy petroleum extracted fromoil sands
Oil sands, tar sands, crude bitumen, or bituminous sands, are a type of unconventional petroleum deposit. Oil sands are either loose sands or partially consolidated sandstone containing a naturally occurring mixture of sand, clay, and wate ...
deposits because sulfur accumulates in the heaviest fractions of hydrocarbons.
Owing to the high sulfur content of the Athabasca Oil Sands, stockpiles of elemental sulfur from this process now exist throughout Alberta
Alberta ( ) is one of the thirteen provinces and territories of Canada. It is part of Western Canada and is one of the three prairie provinces. Alberta is bordered by British Columbia to the west, Saskatchewan to the east, the Northwest Ter ...
, Canada.
Another way of storing sulfur, while reusing it as a valuable material, is as a binder for concrete, the resulting product having many desirable properties (see sulfur concrete).
See also
*Amine treating
Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and c ...
* Hydro-desulfurization
* Crystasulf
*Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
*Acid gas Acid gas is a particular typology of natural gas or any other gas mixture containing significant quantities of hydrogen sulfide (H2S), carbon dioxide (CO2), or similar acidic gases. A gas is determined to be acidic or not after it is mixed with w ...
*Sour gas
Sour gas is natural gas or any other gas containing significant amounts of hydrogen sulfide (H2S).
Natural gas is usually considered sour if there are more than 5.7 milligrams of H2S per cubic meter of natural gas, which is equivalent to approxim ...
References
{{reflist Sulfur Chemical processes German inventions 1883 in science 1883 in Germany