Chlorine-free Germanium Processing on:
[Wikipedia]
[Google]
[Amazon]
 Chlorine-free germanium processing are methods of germanium activation to form useful germanium precursors in a more energy efficient and environmentally friendly way compared to traditional synthetic routes.
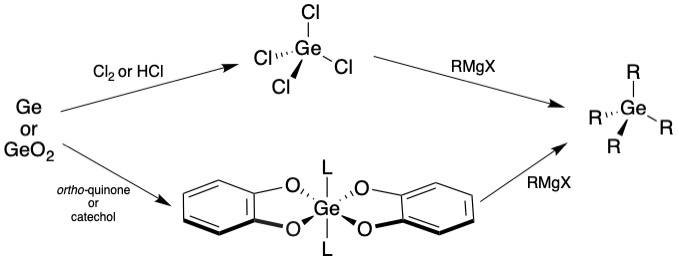
Chlorine-free germanium processing are methods of germanium activation to form useful germanium precursors in a more energy efficient and environmentally friendly way compared to traditional synthetic routes. GeO2 , with HCl Due to the environmental and safety impact of non-recyclable, high energy reactions with HCl , an alternative synthesis of a shelf-stable germanium intermediate precursor without GeR4 without using chloride species was reported, allowing for a much more environmentally friendly and low energy GeO2 , Ge(0) , and even selectively activating germanium in the presence of ZnO ), resulting in products that are bench stable and solid.
Ge(0) , ''ortho''-quinone, and Ge(0) oxidized to Ge(IV) . This reaction was shown to work both at the milligram and the gram scale, proving its efficiency in the bulk scale.
GeO2 with 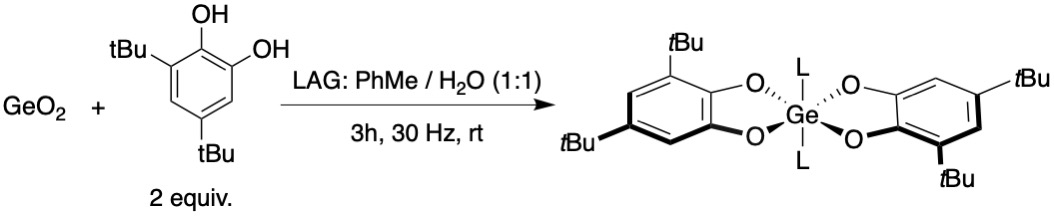
ZnO , contains amounts of GeO2 . Using HCl , the key product of GeCl4 and ZnCl2 byproduct can be produced. The zinc byproduct can be distilled at high temperatures, leaving only germanium tetrachloride. A new method of chlorine-free germanium processing has proven effective in extracting germanium from zinc oxide, giving hope to replace the HCl leaching and distillation process currently employed by industry. In both 1:1 and 1:5 mass ratios of GeO2 and ZnO , germanium oxide was selectively activated by simple addition of catechol, and letting the reaction proceed under the same conditions as the dehydration reaction. The unreacted zinc oxide can be washed away with
GeR4 , of which include, GeH4 ,
GeCl4 , in which the germanium center becomes more sterically hindered over the course of the reaction as ligand exchange of the carbons and the chlorides progresses, making the substitution more difficult.
 The stereochemical selectivity of the substitution reaction is further enforced by the identity of the auxiliary amine ligand. By using a more sterically encumbered amine ligand such as
The stereochemical selectivity of the substitution reaction is further enforced by the identity of the auxiliary amine ligand. By using a more sterically encumbered amine ligand such as BuMgCl . This proves the effect of steric encumbrance on the product of the substitution reaction as the resulting tri-substituted product has the least sterically encumbered oxygen remaining bonded to the catecholate. This reaction pathway could allow new synthetic pathways for more stereo complex and functionalized germanium complexes.
GeH4 , is extremely important in the field of optoelectronics and is a good candidate for vapor deposition to form thin films of germanium. However, germane must be extremely pure to use in such a way, and much research has gone into developing methodologies to prepare and purify germane. Using bis(catecholate) germanium and lithium aluminum hydride (LiAlH4 ) in 
 Chlorine-free germanium processing are methods of germanium activation to form useful germanium precursors in a more energy efficient and environmentally friendly way compared to traditional synthetic routes.
Chlorine-free germanium processing are methods of germanium activation to form useful germanium precursors in a more energy efficient and environmentally friendly way compared to traditional synthetic routes. Germanium tetrachloride
Germanium tetrachloride is a colourless, fuming liquid with a peculiar, acidic odour. It is used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent ...
is a valuable intermediate for the synthesis of many germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
complexes. Normal synthesis of it involves an energy-intensive dehydration of germanium oxide Germanium oxide may refer to:
*Germanium dioxide, GeO2, the best known and most commonly encountered oxide of germanium containing germanium(IV)
*Germanium monoxide
Germanium monoxide, GeO, is a chemical compound of germanium and oxygen
Oxy ...
, hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
, chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
is of interest. In 2017, a synthesis of organogermanes, synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
using zinc oxide
Zinc oxide is an inorganic compound with the formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cemen ...
(Synthesis of organogermanes
Oxidation of germanium metal
Glavinović ''et al.'' have synthesized organogermanes using ''ortho''-quinone, which is bothredox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
"non-innocent" and acts as a pseudo-halide, resulting in an air and moisture stable beige solid. Referring to the scheme below, when pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
(acting as an auxiliary ligand) were milled via liquid assisted grinding in a 1:1 mixture of toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
and water, the resulting organogermane was recrystallized in toluene resulting in 88% yield. In this reaction, the quinone ligands each undergo a two-electron oxidation, resulting in the Dehydration of GeO2
Following a nearly identical reaction scheme as the oxidation of germanium metal with ''ortho-''quinone, dehydration ofcatechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
ligands results in the same product as the oxidation product, with similar yield 74% on milligram scale and 84% on the gram scale. This particular scheme is of much note since the sole byproduct of this reaction is water. These reactions could provide an alternative to normal oxide separations for other metals that are energy intensive and otherwise wasteful.

Extraction from ZnO
Industrially, germanium can be extracted fromdichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
and the bis(catecholate) germanium product recrystallized in cyclohexane. Despite zinc oxide being present in the reaction vessel, the intermediate germanium product yields remain high, being 64 and 66%. This method, as well as other halogen-free germanium extraction methods, make the possibility of halogen free germanium processing a future possibility.
Other auxiliary ligands
The mechanochemical activation of germanium described above can be used with a variety of auxiliary amine-based ligands and not just pyridine as used in the syntheses above. Uni-dentate ligands such as ''N''-methyl imidazole can be used used to create a trans-disposed octahedral germanium product, isostructural to the complexes of both the catechol and ''ortho-''quinone that contain pyridine. However, chelating ligands can be used to form the product with nitrogens cis to each other. For example, in a reaction usingtetramethylethylenediamine
Tetramethylethylenediamine (TMEDA or TEMED) is a chemical compound with the formula (CH3)2NCH2CH2N(CH3)2. This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups. It is a colorless liquid ...
as a chelating bi-dentate diamine affords the cis- product with catechol ligands at the other octahedral binding sites. More research as additionally been done to show that the nitrogen-containing ligands can be biologically active ones which operate at very low reduction potentials. This makes the germanium complexes with those ligands easily reducible and highly nucleophilic, making substitution and activation even easier.
Substitution reactions
Substitutions to form tetraorganogermanes
Reagents and products
The intermediates prepared by the above method are able to easily undergosubstitution reactions
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
with nucleophiles to form tetraorganogermanes, Germane
Germane is the chemical compound with the formula Ge H4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is te ...
. Germane is a key material in optical and electronic device fabrication. These substitution reactions return the original catechol ligand, making this germanium activation process easily recyclable. A solution of 20 equivalents of an alkyl or aryl Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
in tetrahydrofuran, combined with bis(catecholate) complex leads to a homogeneous solution of reagents in THF. Refluxing this solution for 24 hours yields the Grignard product organogermane in relatively high yield across multiple reagents. The figure below shows different reagents used by Glavinović ''et al'', showing the efficacy of the substitution reaction.
Proposed mechanism
The substitution reaction described above is thought to process via a mechanism in which steric strain of the complex is slowly alleviated over the course of the reaction. The first Grignard reagent substitutes the most sterically hindered oxygen position, where the ''t''-butyl group of the catechol ligand isalpha
Alpha (uppercase , lowercase ; grc, ἄλφα, ''álpha'', or ell, άλφα, álfa) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter aleph , whic ...
to the oxygen. The second Grignard reagent substitutes the now uni-dentate catechol-grignard adduct, removing the ligand and resulting in two complete substitutions. Referring to the scheme below, treating intermediate 2 with an additional equivalent of Grignard reagent yields 3 at a faster rate than the rate to make 2, and treatment of 3 with two equivalents of reagent yields 4 at even more quickly. This is starkly different from the substitution reactions of  The stereochemical selectivity of the substitution reaction is further enforced by the identity of the auxiliary amine ligand. By using a more sterically encumbered amine ligand such as
The stereochemical selectivity of the substitution reaction is further enforced by the identity of the auxiliary amine ligand. By using a more sterically encumbered amine ligand such as triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
, a 1.67:1 mixture of dibutyl-germane-η2-catecholate and tributylgermyl-η1-catecholate is produced after substitution with two equivalents of Substitution to form germane
Despite being highly volatile and toxic, germane,dibutyl ether
Dibutyl ether is a chemical compound belonging to the ether family with the molecular formula of . It is colorless, volatile, and flammable liquid and has peculiar ethereal smell.
Liquid dibutyl ether is lighter than water. On the other hand, the ...
with argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
as a carrier gas, the substitution reaction yields high purity germane in the Ar carrier gas with no evolution of volitile Ge byproducts. This reaction pathway for production of germane requires no postsynthetic processing or purification, proving this to be more advantageous than current methods.

References
Wikipedia Student Program Chemical processes {{reflist