|
Chlorine-free Germanium Processing
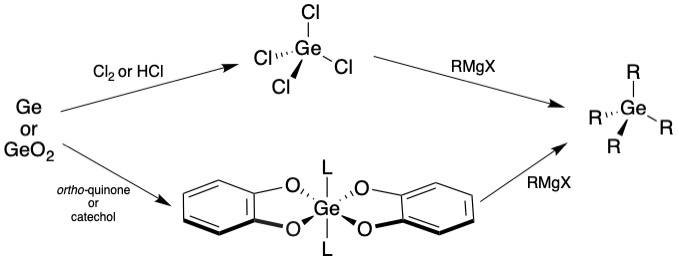
Chlorine-free germanium processing are methods of germanium activation to form useful germanium precursors in a more energy efficient and environmentally friendly way compared to traditional synthetic routes. Germanium tetrachloride is a valuable intermediate for the synthesis of many germanium complexes. Normal synthesis of it involves an energy-intensive dehydration of germanium oxide, GeO2, with hydrogen chloride, HCl Due to the environmental and safety impact of non-recyclable, high energy reactions with HCl, an alternative synthesis of a shelf-stable germanium intermediate precursor without chlorine is of interest. In 2017, a synthesis of organogermanes, GeR4 without using chloride species was reported, allowing for a much more environmentally friendly and low energy synthesis using GeO2, Ge(0), and even selectively activating germanium in the presence of zinc oxide (ZnO), resulting in products that are bench stable and solid. Synthesis of organogermanes Oxidation of germa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Of Germanium Oxide
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mild dehydration can also be caused by immersion diuresis, which may increase risk of decompression sickness in divers. Most people can tolerate a 3-4% decrease in total body water without difficulty or adverse health effects. A 5-8% decrease can cause fatigue and dizziness. Loss of over ten percent of total body water can cause physical and mental deterioration, accompanied by severe thirst. Death occurs at a loss of between fifteen and twenty-five percent of the body water.Ashcroft F, Life Without Water in Life at the Extremes. Berkeley and Los Angeles, 2000, 134-138. Mild dehydration is characterized by thirst and general discomfort and is usually resolved with oral rehydration. Dehydration can cause hypernatremia (high levels of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibutyl Ether
Dibutyl ether is a chemical compound belonging to the ether family with the molecular formula of . It is colorless, volatile, and flammable liquid and has peculiar ethereal smell. Liquid dibutyl ether is lighter than water. On the other hand, the vapor is heavier than air. It is not soluble in water, but it is soluble in acetone and many other organic solvents. Due to this property, dibutyl ether is used as solvent in various chemical reactions and processes. For example, phenyllithium is commercially available as a ca. 1.8M solution in dibutyl ether. Because of the formation of peroxides, it should be protected from heat, light and air. Synthesis Dibutyl ether is obtained from dehydration of 1-butanol with sulfuric acid as a catalyst and dehydrating agent: :2 → + Industrially, dibutyl ether can be obtained by dehydration of 1-butanol on alumina at 300 °C. Reactions This compound is generally stable to oxidation, reduction, and base. Strong acids like HI and HBr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The |
Alpha And Beta Carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule. Numeric locants The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of numeric prefixes to indicate the position of substituents, generally by identifying the parent hydrocarbon chain and assigning the carbon atoms based on their substituents in order of precedence. For example, there are at least two isomers of the linear form of pentanone, a ketone that contains a chain of exactly five carbon atoms. There is an oxygen atom bonded to one of the middle three carbons (if it were bonded to an end carbon, the molecule would be an aldehyde, not a ketone), but it is not clear where it is located. In this example, the carbon atoms are numbered from one to five, which starts at one end and proceeds sequentially along the chain. Now the position of the oxygen atom can be defined as on carbon atom number two, three or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proposed Mechanism Of Germanium Substitution
Proposal(s) or The Proposal may refer to: * Proposal (business) * Research proposal * Proposal (marriage) * Proposition, a proposal in logic and philosophy Arts, entertainment, and media * ''The Proposal'' (album) Films * ''The Proposal'' (1957 film), an Australian television play based on Chekhov's 1890 play * ''The Proposal'' (2001 film), starring Nick Moran, Jennifer Esposito, and Stephen Lang * ''The Proposal'' (2009 film), starring Sandra Bullock and Ryan Reynolds * ''The Proposal'' (2022 film), starring Joe Joseph and Amara Raja * " La propuesta" ("The Proposal"), a short story in the 2014 Argentina anthology film ''Wild Tales'' Literature * '' Proposals (play)'', a 1997 play by Neil Simon * ''The Proposal'' (novel), 1999 and 35th book in the ''Animorphs'' series by K.A. Applegate * ''The Proposal'', alternative title of Chekhov's 1890 play ''A Marriage Proposal'' Television * ''The Proposal'' (American TV series), a 2018 reality dating series * The Proposal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germane
Germane is the chemical compound with the formula Ge H4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride. Occurrence Germane has been detected in the atmosphere of Jupiter. Synthesis Germane is typically prepared by reduction of germanium oxides, notably germanates, with hydride reagents such as sodium borohydride, potassium borohydride, lithium borohydride, lithium aluminium hydride, sodium aluminium hydride. The reaction with borohydrides is catalyzed by various acids and can be carried out in either aqueous or organic solvent. On laboratory scale, germane can be prepared by the reaction of Ge(IV) compounds with these hydride reagents. A typical synthesis involved the reaction of potassium germanate with sodium borohydride. :NaHGeO3 + KBH4 + H2O → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substitution Reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent. A good example of a substitution reaction is halogenation. When chlorine gas (Cl2) is irradiated, some of the molecules are split into two chlorine radicals (Cl•), whose free electrons are strongly n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylethylenediamine
Tetramethylethylenediamine (TMEDA or TEMED) is a chemical compound with the formula (CH3)2NCH2CH2N(CH3)2. This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups. It is a colorless liquid, although old samples often appear yellow. Its odor is similar to that of rotting fish. As a reagent in synthesis TMEDA is widely employed as a ligand for metal ions. It forms stable complexes with many metal halides, e.g. zinc chloride and copper(I) iodide, giving complexes that are soluble in organic solvents. In such complexes, TMEDA serves as a bidentate ligand. TMEDA has an affinity for lithium ions. When mixed with ''n''-butyllithium, TMEDA's nitrogen atoms coordinate to the lithium, forming a cluster of higher reactivity than the tetramer or hexamer that ''n''-butyllithium normally adopts. BuLi/TMEDA is able to metallate or even doubly metallate many substrates including benzene, furan, thiophene, ''N''-alkyl pyrroles, and fer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |