|
1-methylimidazole
1-Methylimidazole or ''N''-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine. Basicity With the ''N''-methyl group, this particular derivative of imidazole cannot tautomerize. It is slightly more basic than imidazole, as indicated by the pKa's of the conjugate acids of 7.0 and 7.4. Methylation also provides a significantly lower melting point, which makes 1-methylimidazole a useful solvent. Synthesis 1-Methylimidazole is prepared mainly by two routes industrially. The main one is acid-catalysed methylation of imidazole by methanol. The second method involves the Radziszewski reaction from glyoxal, formaldehyde, and a mixture of ammonia and methylamine. :(CHO)2 + CH2O + CH3NH2 + NH3 → H2C2N(N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazoles
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Methylimidazole
4-Methylimidazole (4-MeI or 4-MEI) is a heterocyclic organic chemical compound with molecular formula – or . It is formally derived from imidazole through replacement of the hydrogen in position 4 by a methyl group. It is a slightly yellowish solid. 4-MeI may be formed in the browning of certain foods through the Maillard reaction between carbohydrates and amino-containing compounds. In particular, it is found in roasted foods, grilled meats, coffee and in types of caramel coloring produced with ammonia-based processes. It may arise also by fermentation. Preparation and structure 4-MeI may be prepared using the Debus-Radziszewski imidazole synthesis, by reacting methylglyoxal with ammonia and formaldehyde. It may also be prepared by the reaction of hydroxyacetone and formamide in ammonia. Small energy difference separates 4-methylimidazole from its tautomer 5-methylimidazole. Safety Carcinogenicity studies Concern has arisen about the presence of 4-MeI in caramel colo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetism, diamagnetic and has a Magnetic susceptibility, diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodium Carbonyl Chloride
Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful in homogeneous catalysis. Structure The molecule consists of two planar Rh(I) centers linked by two bridging chloride ligands and four CO ligands. X-ray crystallography shows that the two Rh(I) centers are square planar with the dihedral angle of 53° between the two RhCl2 planes. The metals are nonbonding. Synthesis and reactions First prepared by Walter Hieber, it is typically prepared by treating hydrated rhodium trichloride with flowing carbon monoxide, according to this idealized redox equation: :2 RhCl3(H2O)3 + 6 CO → Rh2Cl2(CO)4 + 2 COCl2 + 6 H2O.McCleverty, J. A.; Wilkinson, G. "Dichlorotetracarbonyldirhodium (rhodium carbonyl chloride)" Inorganic Syntheses 1966, volume 8, pp. 211-14. The complex reacts with tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-butyl-3-methylimidazolium Hexafluorophosphate
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobic and non-water-soluble ionic liquid with a melting point of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMIM-BF4, it is one of the most widely studied ionic liquids. It is known to very slowly decompose in the presence of water. Preparation BMIM-PF6 is commercially available. It may be obtained in two steps: BMIM-Cl is synthesized by alkylating 1-methylimidazole with 1-chlorobutane. A metathesis reaction with potassium hexafluorophosphate gives the desired compound; the tetrafluoroborate may be prepared by analogously using potassium tetrafluoroborate Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospher .... : See also * 1-Butyl-3-methylimidazo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
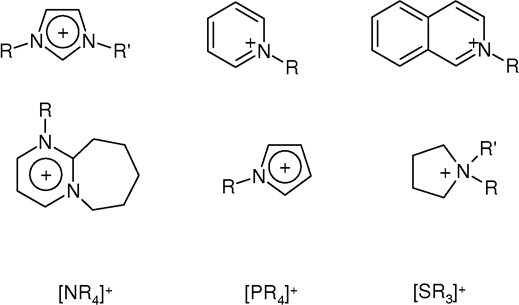
Ionic Liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new cova ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. In vitro methylation of tissue samples is also one method for reducing certain histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals, regulate gene expression, RNA processing and protein function. It has been recognized as a key process underlying epigenetics. Methanogenesis Methanogenesis, the proce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.png)
