Chain initiation on:
[Wikipedia]
[Google]
[Amazon]
Chain-growth polymerization ( AE) or chain-growth polymerisation ( BE) is a
 In 1953,
In 1953,
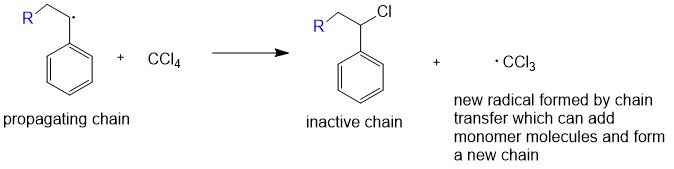 In some chain-growth polymerizations there is also a
In some chain-growth polymerizations there is also a
Internet Encyclopedia of Science
{{DEFAULTSORT:Chain Growth Polymerisation Polymer chemistry Polymerization reactions ja:重合反応#付加縮合
polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
technique where monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
molecules add onto the active site on a growing polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
Chain-growth polymerization involves 3 types of reactions :
# Initiation: An active species I* is formed by some decomposition of an initiator molecule I
# Propagation: The initiator fragment reacts with a monomer M to begin the conversion to the polymer; the center of activity is retained in the adduct. Monomers continue to add in the same way until polymers Pi* are formed with the degree of polymerization i
# Termination: By some reaction generally involving two polymers containing active centers, the growth center is deactivated, resulting in dead polymer
Introduction
Paul Flory
Paul John Flory (June 19, 1910 – September 9, 1985) was an American chemist and Nobel laureate who was known for his work in the field of polymers, or macromolecules. He was a pioneer in understanding the behavior of polymers in solution, and ...
first classified polymerization as "step-growth polymerization
In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Ma ...
" and "chain-growth polymerization". IUPAC recommends to further simplify "chain-growth polymerization" to "chain polymerization". It is a kind of polymerization where an active center (free radical or ion) is formed, and a plurality of monomers can be polymerized together in a short period of time to form a macromolecule having a large molecular weight. In addition to the regenerated active sites of each monomer unit, polymer growth will only occur at one (or possibly more) endpoint.
Many common polymers can be obtained by chain polymerization such as polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
(PE), polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer Propene, propylene.
Polypropylene belongs to the group of polyolefin ...
(PP), polyvinyl chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of ...
(PVC), poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA) is a synthetic polymer derived from methyl methacrylate. It is a transparent thermoplastic, used as an engineering plastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and bran ...
(PMMA), polyacrylonitrile
Polyacrylonitrile (PAN) is a synthetic, semicrystalline organic polymer resin, with the linear formula (CH2CHCN)n. Almost all PAN resins are copolymers with acrylonitrile as the main monomer. PAN is used to produce large variety of products in ...
(PAN), polyvinyl acetate
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue (a term that may also refer to other types of glues), PVA glue, white glue, carpenter's glue, school glue, or Elmer's Glue in the US, is a widely available adh ...
(PVA).
Typically, chain-growth polymerization can be understood with the chemical equation:
:
In this equation, P is the polymer while x represents degree of polymerization, * means active center of chain-growth polymerization, M is the monomer which will react with active center, and L may be a low-molar-mass by-product obtained during chain propagation. For most chain-growth polymerizations, there is no by-product L formed. However there are some exceptions, such as the polymerization of amino acid ''N''-carboxyanhydrides to oxazolidine-2,5-diones.
This type of polymerization is described as "chain" or "chain-growth" because the reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
is a chemical chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
with an initiation step in which an active center is formed, followed by a rapid sequence of chain propagation
In chemistry, chain propagation (sometimes just referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a Chain reaction#Chemical chain reactions, chemical chain reaction. For exa ...
steps in which the polymer molecule grows by addition of one monomer molecule to the active center in each step. The word "chain" here does not refer to the fact that polymer molecules form long chains. Some polymers are formed instead by a second type of mechanism known as step-growth polymerization
In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Ma ...
without rapid chain propagation steps.
Reaction steps
All chain-growth polymerization reactions must include chain initiation and chain propagation. Chain transfer and chain termination steps also occur in many but not all chain-growth polymerizations.Chain initiation
Chain initiation is the initial generation of a chain carrier, which is an intermediate such as a radical or an ion which can continue the reaction by chain propagation. Initiation steps are classified according to the way that energy is provided: thermal initiation, high energy initiation, and chemical initiation, etc. Thermal initiation uses molecular thermal motion to dissociate a molecule and form active centers. High energy initiation refers to the generation of chain carriers by radiation. Chemical initiation is due to a chemical initiator. For the case ofradical polymerization
In polymer chemistry, radical polymerization (RP) is a method of polymerization by which a polymer forms by the successive addition of a radical to building blocks ( repeat units). Radicals can be formed by a number of different mechanisms, usu ...
as an example, chain initiation involves the dissociation of a radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical in ...
molecule (I) which is easily dissociated by heat or light into two free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired electron, unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemical reaction, chemi ...
(2 R°). Each radical R° then adds a first monomer molecule (M) to start a chain which terminates with a monomer activated by the presence of an unpaired electron (RM1°).
* I → 2 R°
* R° + M → RM1°
Chain propagation
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
defines chain propagation
In chemistry, chain propagation (sometimes just referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a Chain reaction#Chemical chain reactions, chemical chain reaction. For exa ...
as a reaction of an active center on the growing polymer molecule, which adds one monomer molecule to form a new polymer molecule (RM1°) one repeat unit longer.
For radical polymerization, the active center remains an atom with an unpaired electron. The addition of the second monomer and a typical later addition step are
* RM1° + M → RM2°
* ...............
* RMn° + M → RMn+1°
For some polymers, chains of over 1000 monomer units can be formed in milliseconds.
Chain termination
In achain termination
In polymer chemistry, chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
Mechanisms of termination ...
step, the active center disappears, resulting in the termination of chain propagation. This is different from chain transfer in which the active center only shifts to another molecule but does not disappear.
For radical polymerization, termination involves a reaction of two growing polymer chains to eliminate the unpaired electrons of both chains. There are two possibilities.
1. Recombination is the reaction of the unpaired electrons of two chains to form a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
between them. The product is a single polymer molecule with the combined length of the two reactant chains:
* RMn° + RMm° → Pn+m
2. Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
is the transfer of a hydrogen atom from one chain to the other, so that the two product chain molecules are unchanged in length but are no longer free radicals:
* RMn° + RMm° → Pn + Pm
Initiation, propagation and termination steps also occur in chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
s of smaller molecules. This is not true of the chain transfer and branching steps considered next.
Chain transfer
 In some chain-growth polymerizations there is also a
In some chain-growth polymerizations there is also a chain transfer
In polymer chemistry, chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule:
\ce^\bullet + \ce^\bullet
where • is the active center, P is the initial polymer chain, X i ...
step, in which the growing polymer chain RMn° takes an atom X from an inactive molecule XY, terminating the growth of the polymer chain: RMn° + XY → RMnX + Y°. The Y fragment ls a new active center which adds more monomer M to form a new growing chain YMn°. This can happen in free radical polymerization for chains RMn°, in ionic polymerization for chains RMn+ or RMn–, or in coordination polymerization. In most cases chain transfer will generate a by-product and decrease the molar mass of the final polymer.
Chain transfer to polymer: Branching
Another possibility is chain transfer to a second polymer molecule, result in the formation of a product macromolecule with a branched structure. In this case the growing chain takes an atom X from a second polymer chain whose growth had been completed. The growth of the first polymer chain is completed by the transfer of atom X. However the second molecule loses an atom X from the interior of its polymer chain to form a reactive radical (or ion) which can add more monomer molecules. This results in the addition of a branch orside chain
In organic chemistry and biochemistry, a side chain is a substituent, chemical group that is attached to a core part of the molecule called the "main chain" or backbone chain, backbone. The side chain is a hydrocarbon branching element of a mo ...
and the formation of a product macromolecule with a branched structure.
Classes of chain-growth polymerization
TheInternational Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) recommends definitions for several classes of chain-growth polymerization.
Radical polymerization
Based on the IUPAC definition,radical polymerization
In polymer chemistry, radical polymerization (RP) is a method of polymerization by which a polymer forms by the successive addition of a radical to building blocks ( repeat units). Radicals can be formed by a number of different mechanisms, usu ...
is a chain polymerization in which the kinetic-chain carriers are radicals
Radical (from Latin: ', root) may refer to:
Politics and ideology Politics
*Classical radicalism, the Radical Movement that began in late 18th century Britain and spread to continental Europe and Latin America in the 19th century
*Radical politics ...
. Usually, the growing chain end bears an unpaired electron. Free radicals can be initiated by many methods such as heating, redox reactions, ultraviolet radiation, high energy irradiation, electrolysis, sonication, and plasma.
Free radical polymerization is very important in polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
chemistry. It is one of the most developed methods in chain-growth polymerization. Currently, most polymers in our daily life are synthesized by free radical polymerization, including polyethylene, polystyrene, polyvinyl chloride, polymethyl methacrylate, polyacrylonitrile, polyvinyl acetate
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue (a term that may also refer to other types of glues), PVA glue, white glue, carpenter's glue, school glue, or Elmer's Glue in the US, is a widely available adh ...
, styrene butadiene rubber, nitrile rubber, neoprene, etc.
Ionic polymerization
Ionic polymerization is a chain polymerization in which the kinetic-chain carriers are ions or ion pairs. It can be further divided into anionic polymerization andcationic polymerization
In polymer chemistry, cationic polymerization is a type of Chain growth polymerisation, chain growth polymerization in which a cationic initiator transfers charge to a monomer, which then becomes reactive. This reactive monomer goes on to react si ...
.
Ionic polymerization generates many polymers used in daily life, such as butyl rubber, polyisobutylene, polyphenylene, polyoxymethylene, polysiloxane, polyethylene oxide, high density polyethylene, isotactic polypropylene, butadiene rubber, etc. Living anionic polymerization was developed in the 1950s. The chain will remain active indefinitely unless the reaction is transferred or terminated deliberately, which allows the control of molar weight and dispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsi ...
(or polydispersity index, PDI).
Coordination polymerization
Coordination polymerization is a chain polymerization that involves the preliminary coordination of a monomer molecule with a chain carrier. The monomer is first coordinated with the transition metal active center, and then the activated monomer is inserted into the transition metal-carbon bond for chain growth. In some cases, coordination polymerization is also called insertion polymerization or complexing polymerization. Advanced coordination polymerizations can control thetacticity
Tacticity (from , "relating to arrangement or order") is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical properties of the polymer. The ...
, molecular weight and PDI of the polymer effectively. In addition, the racemic mixture of the chiral metallocene can be separated into its enantiomers. The oligomerization reaction produces an optically active branched olefin using an optically active catalyst.
Living polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transf ...
was first described by Michael Szwarc in 1956. It is defined as a chain polymerization from which chain transfer and chain termination are absent. In the absence of chain-transfer and chain termination, the monomer in the system is consumed and the polymerization stops but the polymer chain remains active. If new monomer is added, the polymerization can proceed.
Due to the low PDI and predictable molecular weight, living polymerization is at the forefront of polymer research. It can be further divided into living free radical polymerization, living ionic polymerization and living ring-opening metathesis polymerization, etc.
Ring-opening polymerization
Ring-opening polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anion ...
is defined as a polymerization in which a cyclic monomer yields a monomeric unit which is acyclic or contains fewer cycles than the monomer. Generally, the ring-opening polymerization is carried out under mild conditions, and the by-product is less than in the polycondensation reaction. A high molecular weight polymer is easily obtained.
Common ring-opening polymerization products includes polypropylene oxide
Polypropylene glycol or polypropylene oxide is the polymer (or macromolecule) of propylene glycol. Chemically it is a polyether, and, more generally speaking, it's a polyalkylene glycol (PAG) H S Code 3907.2000. The term polypropylene glycol or PPG ...
, polytetrahydrofuran
Polytetrahydrofuran, also called poly(tetramethylene ether) glycol or poly(tetramethylene oxide), is a collection of chemical compounds with formula or HO((CH2)4O-)''n''-H. The material is a mixture of polyether diols terminated with alcohol gro ...
, , polyoxymethylene
Polyoxymethylene (POM), also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction, and excellent dimensional stability. Short-chained POM (chain length ...
, polycaprolactam and polysiloxane
In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants ...
.
Reversible-deactivation polymerization
Reversible-deactivation polymerization
In polymer chemistry, reversible-deactivation polymerization (RDP) is a form of polymerization propagated by chain carriers, some of which at any instant are held in a state of dormancy through an equilibrium process involving other species.
A ...
is defined as a chain polymerization propagated by chain carriers that are deactivated reversibly, bringing them into one or more active-dormant equilibria. An example of a reversible-deactivation polymerization is group-transfer polymerization.
Comparison with step-growth polymerization
Polymers were first classified according to polymerization method byWallace Carothers
Wallace Hume Carothers (; April 27, 1896 – April 29, 1937) was an American chemist, inventor, and the leader of organic chemistry at DuPont, who was credited with the invention of nylon.
Carothers was a group leader at the DuPont Experimen ...
in 1929, who introduced the terms addition polymer
In polymer chemistry, an addition polymer is a polymer that forms by simple linking of monomers ''without'' the co-generation of other products. Addition polymerization differs from condensation polymerization, which ''does'' co-generate a product ...
and condensation polymer
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Natural proteins as well as s ...
to describe polymers made by addition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
s and condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
s respectively. However this classification is inadequate to describe a polymer which can be made by either type of reaction, for example nylon 6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the compari ...
which can be made either by addition of a cyclic monomer or by condensation of a linear monomer.
Flory revised the classification to chain-growth polymerization and step-growth polymerization
In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Ma ...
, based on polymerization mechanisms rather than polymer structures. IUPAC now recommends that the names of step-growth polymerization and chain-growth polymerization be further simplified to polycondensation (or polyaddition if no low-molar-mass by-product is formed when a monomer is added) and chain polymerization.
Most polymerizations are either chain-growth or step-growth reactions. Chain-growth includes both initiation and propagation steps (at least), and the propagation of chain-growth polymers proceeds by the addition of monomers to a growing polymer with an active centre. In contrast step-growth polymerization involves only one type of step, and macromolecules can grow by reaction steps between any two molecular species: two monomers, a monomer and a growing chain, or two growing chains. In step growth, the monomers will initially form dimers, trimers, etc. which later react to form long chain polymers.
In chain-growth polymerization, a growing macromolecule increases in size rapidly once its growth is initiated. When a macromolecule stops growing it generally will add no more monomers. In step-growth polymerization on the other hand, a single polymer molecule can grow over the course of the whole reaction.
In chain-growth polymerization, long macromolecules with high molecular weight are formed when only a small fraction of monomer has reacted. Monomers are consumed steadily over the course of the whole reaction, but the degree of polymerization can increase very quickly after chain initiation. However in step-growth polymerization the monomer is consumed very quickly to dimer, trimer and oligomer. The degree of polymerization increases steadily during the whole polymerization process.
The type of polymerization of a given monomer usually depends on the functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s present, and sometimes also on whether the monomer is linear or cyclic. Chain-growth polymers are usually addition polymers by Carothers' definition. They are typically formed by addition reactions of C=C bonds in the monomer backbone, which contains only carbon-carbon bonds. Another possibility is ring-opening polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anion ...
, as for the chain-growth polymerization of tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
or of polycaprolactone
Polycaprolactone (PCL) is a synthetic, semi-crystalline, biodegradable polyester with a melting point of about 60 °C and a glass transition temperature of about −60 °C. The most common use of polycaprolactone is in the production ...
(see Introduction above).
Step-growth polymers are typically condensation polymers in which an elimination product as such as H2O are formed. Examples are polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made throug ...
s, polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...
s, polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
s, polyimide
Polyimide (sometimes abbreviated PI) is a monomer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, suc ...
s, polysiloxane
In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants ...
s and polysulfone
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl- SO2-aryl subunit. Due to the high cost of raw material ...
s. If no elimination product is formed, then the polymer is an addition polymer, such as a polyurethane
Polyurethane (; often abbreviated PUR and PU) is a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term ...
or a poly(phenylene oxide). Chain-growth polymerization with a low-molar-mass by-product during chain growth is described by IUPAC as "condensative chain polymerization".
Compared to step-growth polymerization, living chain-growth polymerization shows low molar mass dispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsi ...
(or PDI), predictable molar mass distribution
In polymer chemistry, the molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species () and the molar mass () of that species. In linear polymers, the individual polym ...
and controllable conformation. Generally, polycondensation proceeds in a step-growth polymerization mode.
Application
Chain polymerization products are widely used in many aspects of life, including electronic devices, food packaging, catalyst carriers, medical materials, etc. At present, the world's highest yielding polymers such as polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), etc. can be obtained by chain polymerization. In addition, some carbon nanotube polymer is used for electronical devices. Controlled living chain-growth conjugated polymerization will also enable the synthesis of well-defined advanced structures, including block copolymers. Their industrial applications extend to water purification, biomedical devices and sensors.References
External links
Internet Encyclopedia of Science
{{DEFAULTSORT:Chain Growth Polymerisation Polymer chemistry Polymerization reactions ja:重合反応#付加縮合