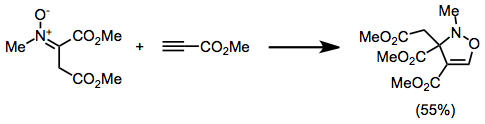|
Oxazolidine
An oxazolidine is a five-membered ring compound consisting of three carbon atoms, a nitrogen atom and an oxygen atom. The O atom and NH group are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon atom between the O and N atoms (or it would be an ''isoxazolidine''). All of the carbon atoms in oxazolidines are reduced (compare to oxazole and oxazoline). Some of their derivatives, the oxazolidinediones, are used as anticonvulsants. Oxazolidines were first synthesized over 100 years ago. Monooxazolidines Oxazolidines that are the precursor to bisoxazolidines are in effect mono-oxazolidines. They are also used as moisture scavengers in polyurethane and other systems. Dioxooxazolidines Oxazolidines where the carbon centers at the 1 and 3 positions are carbonyls are called dioxooxazolidines. Some of these are commercial fungicides including chlozolinate, vinclozolin, and famoxadone. Bisoxazolidines Bisoxazolidines are chemical compounds tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazolidine
An oxazolidine is a five-membered ring compound consisting of three carbon atoms, a nitrogen atom and an oxygen atom. The O atom and NH group are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon atom between the O and N atoms (or it would be an ''isoxazolidine''). All of the carbon atoms in oxazolidines are reduced (compare to oxazole and oxazoline). Some of their derivatives, the 2,4-Oxazolidinedione, oxazolidinediones, are used as anticonvulsants. Oxazolidines were first synthesized over 100 years ago. Monooxazolidines Oxazolidines that are the precursor to bisoxazolidines are in effect mono-oxazolidines. They are also used as moisture scavengers in polyurethane and other systems. Dioxooxazolidines Oxazolidines where the carbon centers at the 1 and 3 positions are carbonyl group, carbonyls are called dioxooxazolidines. Some of these are commercial fungicides including chlozolinate, vinclozolin, and famoxadone. Bisoxazolidines Bisoxazol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,4-Oxazolidinedione
2,4-Oxazolidinedione is an organic compound with the formula HN(CO)2OCH2. It is a white solid. The parent ring is not particularly important, but this core structure is found in a variety anticonvulsant drugs. The parent compound is obtained by treating chloroacetamide with bicarbonate. File:Dimethadione.svg, Dimethadione File:Ethadione.svg, Ethadione File:Paramethadione.svg, Paramethadione File:Trimethadione.svg, Trimethadione See also * Glycine N-carboxyanhydride, the parent 2,5-oxazolidinedione References Anticonvulsants Oxazolidinediones, {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticonvulsant
Anticonvulsants (also known as antiepileptic drugs or recently as antiseizure drugs) are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain. Conventional antiepileptic drugs may block sodium channels or enhance γ-aminobutyric acid ( GABA) function. Several antiepileptic drugs have multiple or uncertain mechanisms of action. Next to the voltage-gated sodium channels and components of the GABA system, their targets include GABAA receptors, the GAT-1 GABA transporter, and GABA transaminase. Additional targets include voltage-gated calcium channels, SV2A, and α2δ. By blocking sodium or ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond. Oxazoline itself has no applications however oxazolines have been widely investigated for potential applications. These applications include use as ligands in asymmetric catalysis, as protecting groups for carboxylic acids and increasingly as monomers for the production of polymers. Isomers Synthesis The synthesis of 2-oxazoline rings is well established and in general proceeds via the cyclisation of a 2-amino alcohol (typically obtained by the reduction of an amino acid) with a suitable functional group. The overall mechanism is usuall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinclozolin
Vinclozolin (trade names Ronilan, Curalan, Vorlan, Touche) is a common Dicarboximide fungicides, dicarboximide fungicide used to control diseases, such as blights, rots and molds in vineyards, and on fruits and vegetables such as raspberries, lettuce, kiwi, snap beans, and onions. It is also used on turf on golf courses. Two common fungi that vinclozolin is used to protect crops against are ''Botrytis cinerea'' and ''Sclerotinia sclerotiorum''. First registered in 1981, vinclozolin is widely used but its overall application has declined. As a pesticide, vinclozolin is regulated by the United States Environmental Protection Agency (U.S. EPA). In addition to these restrictions within the United States, as of 2006 the use of this pesticide was banned in several countries, including Denmark, Finland, Norway, and Sweden. It has gone through a series of tests and regulations in order to evaluate the risks and hazards to the environment and animals. Among the research, a main finding is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazole
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a p''K''a of 0.8, compared to 7 for imidazole. Preparation Classical oxazole synthetic methods in organic chemistry are * the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones * the Fischer oxazole synthesis from cyanohydrins and aldehydes * the Bredereck reaction with α-haloketones and formamide * the Van Leusen reaction with aldehydes and TosMIC Other methods: * Oxazolines can also be obtained from cycloisomerization of certain propargyl amides. In one study oxazoles were prepared via a one-pot synthesis consisting of the condensation of propargyl amine and benzoyl chloride to the amide, followed by a Sonogashira coupling of the terminal alkyne end with another equivalent of benzoylchlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond. Oxazoline itself has no applications however oxazolines have been widely investigated for potential applications. These applications include use as ligands in asymmetric catalysis, as protecting groups for carboxylic acids and increasingly as monomers for the production of polymers. Isomers Synthesis The synthesis of 2-oxazoline rings is well established and in general proceeds via the cyclisation of a 2-amino alcohol (typically obtained by the reduction of an amino acid) with a suitable functional group. The overall mechanism is usuall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Famoxadone
Famoxadone is a fungicide to protect agricultural products against various fungal diseases on fruiting vegetables, tomatoes, potatoes, curcurbits, lettuce and grapes.Famoxadone Pesticide Fact Sheet It is used in combination with cymoxanil. Famoxadone is a QI, albeit with a chemistry different from most QIs. (It is an [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazole
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole. Occurrence Isoxazole rings are found in some natural products, such as ibotenic acid and muscimol. Synthesis Isoxazole can be synthesised via a variety of methods. Examples include via a 1,3-dipolar cycloaddition of nitrile oxides with alkynes; or the reaction of hydroxylamine with 1,3-diketones or derivatives of propiolic acid. Photochemistry The photolysis of isoxazole was first reported in 1966. Due to the weak N-O bond, the isoxazole ring tends to collapse under UV irradiation, rearranging to oxazole through azirine intermediate. Meanwhile, the azirine intermediate can react with nucleophiles, especially carboxylic acids. Given the photoreactions, isoxazole group is developed as a native photo-cross-linker for photoaffinity labeling and chemoproteomic studies. Pharmaceuticals and h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrone-olefin (3+2) Cycloaddition
The nitrone-olefin (3+2) cycloaddition reaction is the combination of a nitrone with an alkene or alkyne to generate an isoxazoline or isoxazolidine via a +2cycloaddition process. This reaction is a 1,3-dipolar cycloaddition, in which the nitrone acts as the 1,3-dipole, and the alkene or alkyne as the dipolarophile. Mechanism and stereochemistry When nitrones are combined with either alkenes or alkynes, +2cycloaddition leads to the formation of a new C–C bond and a new C–O bond. The cycloadditions is stereospecific with respect to the configuration of the alkene; however, diastereoselectivity in reactions of C-substituted nitrones is often low. Regioselectivity is controlled by the dominant frontier orbitals interacting during the reaction, and substrates with electronically distinct substituents tend to react with high regioselectivity. Intramolecular versions of the reaction have been used to synthesize complex polyclic carbon frameworks. Reduction of the N–O linkage l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group. Oxazoles are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazoles, with the thiazole sulfur replaced by nitrogen. Thiazole rings are planar and aromatic. Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazoles and have therefore greater aromaticity. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR spectroscopy (between 7.27 and 8.77 ppm), clearly indicating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |