Catalytic Hydroboration Regioselectivity on:
[Wikipedia]
[Google]
[Amazon]
 Catalysis () is the process of increasing the
Catalysis () is the process of increasing the

 Heterogeneous catalysts act in a different
Heterogeneous catalysts act in a different
 Estimates are that 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. In 2005, catalytic processes generated about $900 billion in products worldwide. The global demand for catalysts in 2014 was estimated at US$33.5 billion. Catalysis is so pervasive that subareas are not readily classified. Some areas of particular concentration are surveyed below.
Estimates are that 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. In 2005, catalytic processes generated about $900 billion in products worldwide. The global demand for catalysts in 2014 was estimated at US$33.5 billion. Catalysis is so pervasive that subareas are not readily classified. Some areas of particular concentration are surveyed below.
 Some of the largest-scale chemicals are produced via catalytic oxidation, often using
Some of the largest-scale chemicals are produced via catalytic oxidation, often using
Science Aid: Catalysts
Page for high school level science
W.A. Herrmann Technische UniversitÀt presentation
Alumite Catalyst, Kameyama-Sakurai Laboratory, Japan
Inorganic Chemistry and Catalysis Group, Utrecht University, The Netherlands
Carbons & Catalysts Group, University of Concepcion, Chile
Center for Enabling New Technologies Through Catalysis, An NSF Center for Chemical Innovation, USA
"Bubbles turn on chemical catalysts"
Science News magazine online, April 6, 2009. {{Authority control Chemical kinetics Articles containing video clips
rate
Rate or rates may refer to:
Finance
* Rates (tax), a type of taxation system in the United Kingdom used to fund local government
* Exchange rate, rate at which one currency will be exchanged for another
Mathematics and science
* Rate (mathema ...
of a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.
Catalysis may be classified as either homogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
, whose components are not in the same phase. Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s and other biocatalysts are often considered as a third category.
Catalysis is ubiquitous in chemical industry
The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials (oil, natural gas, air, water, metals, and minerals) into more than 70,000 different products. The ...
of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture.
The term "catalyst" is derived from Greek , ''kataluein'', meaning "loosen" or "untie". The concept of catalysis was invented by chemist Elizabeth Fulhame, based on her novel work in oxidation-reduction experiments.
General principles
Illustration
Illustrative is thedisproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can b ...
of hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%â ...
to water and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
:
:2 HO â 2 HO + O
This reaction proceeds because the reaction products are more stable than the starting material. The uncatalyzed reaction is slow. In fact, the decomposition of hydrogen peroxide is so slow that hydrogen peroxide solutions are commercially available. This reaction is strongly affected by catalysts such as manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell ...
, or the enzyme peroxidase in organisms. Upon the addition of a small amount of manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell ...
, the hydrogen peroxide reacts rapidly. This effect is readily seen by the effervescence
Effervescence is the escape of gas from an aqueous solution and the foaming or fizzing that results from that release. The word effervescence is derived from the Latin verb ''fervere'' (to boil), preceded by the adverb ''ex''. It has the same lin ...
of oxygen. The manganese dioxide is not consumed in the reaction, and thus may be recovered unchanged, and re-used indefinitely. Accordingly, manganese dioxide ''catalyzes'' this reaction.
Units
TheSI derived unit
SI derived units are units of measurement derived from the
seven base units specified by the International System of Units (SI). They can be expressed as a product (or ratio) of one or more of the base units, possibly scaled by an appropriate po ...
for measuring the catalytic activity of a catalyst is the katal, which is quantified in moles per second. The productivity of a catalyst can be described by the turnover number Turnover number has two different meanings:
In enzymology, turnover number (also termed ''k''cat) is defined as the maximum number of chemical conversions of substrate molecules per second that a single active site will execute for a given enzyme ...
(or TON) and the catalytic activity by the ''turn over frequency'' (TOF), which is the TON per time unit. The biochemical equivalent is the enzyme unit. For more information on the efficiency of enzymatic catalysis, see the article on ''enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
''.
Catalytic reaction mechanisms
In general, chemical reactions occur faster in the presence of a catalyst because the catalyst provides an alternative reaction pathway - or mechanism - with a loweractivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
than the non-catalyzed mechanism. In catalyzed mechanisms, the catalyst usually reacts to form an intermediate, which then regenerates the original catalyst in the process.
As a simple example in the gas phase, the reaction 2 SO2 + O2 â 2 SO3 can be catalyzed by adding nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
. The reaction occurs in two steps:
: 2NO + O2 â 2NO2 (rate-determining)
: NO2 + SO2 â NO + SO3 (fast)
The NO catalyst is regenerated. The overall rate is the rate of the slow step
:v = 2k1 Osup>2 2
An example of heterogeneous catalysis is the reaction of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
on the surface of titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insolubl ...
(TiO, or ''titania'') to produce water. Scanning tunneling microscopy showed that the molecules undergo adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
and dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts) ...
. The dissociated, surface-bound O and H atoms diffuse together. The intermediate reaction states are: HO, HO, then HO and the reaction product ( water molecule dimers), after which the water molecule desorbs from the catalyst surface.
Reaction energetics
Catalysts enable pathways that differ from the uncatalyzed reactions. These pathways have loweractivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
. Consequently, more molecular collisions have the energy needed to reach the transition state. Hence, catalysts can enable reactions that would otherwise be blocked or slowed by a kinetic barrier. The catalyst may increase the reaction rate or selectivity, or enable the reaction at lower temperatures. This effect can be illustrated with an energy profile
In theoretical chemistry, an energy profile is a theoretical representation of a chemical reaction or Chemical process, process as a single energetic pathway as the reactants are transformed into Product (chemistry), products. This pathway runs al ...
diagram.
In the catalyzed elementary reaction, catalysts do not change the extent of a reaction: they have no effect on the chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the sy ...
of a reaction. The ratio of the forward and the reverse reaction rates is unaffected (see also thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
). The second law of thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and Energy transformation, energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects ( ...
describes why a catalyst does not change the chemical equilibrium of a reaction. Suppose there was such a catalyst that shifted an equilibrium. Introducing the catalyst to the system would result in a reaction to move to the new equilibrium, producing energy. Production of energy is a necessary result since reactions are spontaneous only if Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
is produced, and if there is no energy barrier, there is no need for a catalyst. Then, removing the catalyst would also result in a reaction, producing energy; i.e. the addition and its reverse process, removal, would both produce energy. Thus, a catalyst that could change the equilibrium would be a perpetual motion machine, a contradiction to the laws of thermodynamics. Thus, catalyst does not alter the equilibrium constant. (A catalyst can however change the equilibrium concentrations by reacting in a subsequent step. It is then consumed as the reaction proceeds, and thus it is also a reactant. Illustrative is the base-catalyzed hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s, where the produced carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
immediately reacts with the base catalyst and thus the reaction equilibrium is shifted towards hydrolysis.)
The catalyst stabilizes the transition state more than it stabilizes the starting material. It decreases the kinetic barrier by decreasing the ''difference'' in energy between starting material and the transition state. It does not change the energy difference between starting materials and products (thermodynamic barrier), or the available energy (this is provided by the environment as heat or light).
Related concepts
Some so-called catalysts are really precatalysts. Precatalysts convert to catalysts in the reaction. For example, Wilkinson's catalyst RhCl(PPh) loses one triphenylphosphine ligand before entering the true catalytic cycle. Precatalysts are easier to store but are easily activated in situ. Because of this preactivation step, many catalytic reactions involve an induction period. In cooperative catalysis, chemical species that improve catalytic activity are called cocatalysts or promoters. Intandem catalysis
Concurrent tandem catalysis (CTC) is a technique in chemistry where multiple catalysts (usually two) produce a product otherwise not accessible by a single catalyst. It is usually practiced as homogeneous catalysis.
Scheme 1 illustrates this proces ...
two or more different catalysts are coupled in a one-pot reaction.
In autocatalysis, the catalyst ''is'' a product of the overall reaction, in contrast to all other types of catalysis considered in this article. The simplest example of autocatalysis is a reaction of type A + B â 2 B, in one or in several steps. The overall reaction is just A â B, so that B is a product. But since B is also a reactant, it may be present in the rate equation and affect the reaction rate. As the reaction proceeds, the concentration of B increases and can accelerate the reaction as a catalyst. In effect, the reaction accelerates itself or is autocatalyzed. An example is the hydrolysis of an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
such as aspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat inc ...
to a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
and an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
. In the absence of added acid catalysts, the carboxylic acid product catalyzes the hydrolysis.
A true catalyst can work in tandem with a sacrificial catalyst
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, material ...
. The true catalyst is consumed in the elementary reaction and turned into a deactivated form.
The sacrificial catalyst regenerates the true catalyst for another cycle. The sacrificial catalyst is consumed in the reaction, and as such, it is not really a catalyst, but a reagent. For example, osmium tetroxide
Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the ...
(OsO4) is a good reagent for dihydroxylation, but it is highly toxic and expensive. In Upjohn dihydroxylation, the sacrificial catalyst N-methylmorpholine N-oxide
''N''-Methylmorpholine ''N''-oxide (more correctly 4-methylmorpholine 4-oxide), NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in ...
(NMMO) regenerates OsO4, and only catalytic quantities of OsO4 are needed.
Classification
Catalysis may be classified as either homogeneous or heterogeneous. A homogeneous catalysis is one whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant's molecules. A heterogeneous catalysis is one where the reaction components are not in the same phase.Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s and other biocatalysts are often considered as a third category. Similar mechanistic principles apply to heterogeneous, homogeneous, and biocatalysis.
Heterogeneous catalysis

phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
* Phase space, a mathematic ...
than the reactants
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
. Most heterogeneous catalysts are solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
s that act on substrates in a liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
or gaseous reaction mixture
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
. Important heterogeneous catalysts include zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula y where is either a metal ion or H+. These pos ...
s, alumina, higher-order oxides, graphitic carbon, transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2â (molecular) ion. with oxygen in the oxidation state of â2. Most of the E ...
s, metals such as Raney nickel for hydrogenation, and vanadium(V) oxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because o ...
for oxidation of sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
into sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide, also known as ''nisso sulfan'') is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an ind ...
by the so-called contact process
The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arsenic ...
.
Diverse mechanisms for reactions on surfaces are known, depending on how the adsorption takes place ( Langmuir-Hinshelwood, Eley-Rideal, and Mars- van Krevelen). The total surface area of a solid has an important effect on the reaction rate. The smaller the catalyst particle size, the larger the surface area for a given mass of particles.
A heterogeneous catalyst has active sites, which are the atoms or crystal faces where the reaction actually occurs. Depending on the mechanism, the active site may be either a planar exposed metal surface, a crystal edge with imperfect metal valence, or a complicated combination of the two. Thus, not only most of the volume but also most of the surface of a heterogeneous catalyst may be catalytically inactive. Finding out the nature of the active site requires technically challenging research. Thus, empirical research for finding out new metal combinations for catalysis continues.
For example, in the Haber process, finely divided iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
serves as a catalyst for the synthesis of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
from nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
. The reacting gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
es adsorb
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fl ...
onto active sites on the iron particles. Once physically adsorbed, the reagents undergo chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like cor ...
that results in dissociation into adsorbed atomic species, and new bonds between the resulting fragments form in part due to their closeness. In this way the particularly strong triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
in nitrogen is broken, which would be extremely uncommon in the gas phase due to its high activation energy. Thus, the activation energy of the overall reaction is lowered, and the rate of reaction increases. Another place where a heterogeneous catalyst is applied is in the oxidation of sulfur dioxide on vanadium(V) oxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because o ...
for the production of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
.
Heterogeneous catalysts are typically " supported," which means that the catalyst is dispersed on a second material that enhances the effectiveness or minimizes its cost. Supports prevent or minimize agglomeration and sintering of small catalyst particles, exposing more surface area, thus catalysts have a higher specific activity (per gram) on support. Sometimes the support is merely a surface on which the catalyst is spread to increase the surface area. More often, the support and the catalyst interact, affecting the catalytic reaction. Supports can also be used in nanoparticle synthesis by providing sites for individual molecules of catalyst to chemically bind. Supports are porous materials with a high surface area, most commonly alumina, zeolites or various kinds of activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
. Specialized supports include silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
, titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insolubl ...
, calcium carbonate, and barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium an ...
.
In slurry reactions, heterogeneous catalysts can be lost by dissolving.
Many heterogeneous catalysts are in fact nanomaterials. Nanomaterial-based catalyst Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalyst ...
s with enzyme-mimicking activities are collectively called as nanozymes
An artificial enzyme is a synthetic organic molecule or ion that recreates one or more functions of an enzyme. It seeks to deliver catalysis at rates and selectivity observed in naturally occurring enzymes.
History
Enzyme catalysis of chemical re ...
.
Electrocatalysts
In the context ofelectrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
, specifically in fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
engineering, various metal-containing catalysts are used to enhance the rates of the half reactions that comprise the fuel cell. One common type of fuel cell electrocatalyst is based upon nanoparticles
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 1 ...
of platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
that are supported on slightly larger carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
particles. When in contact with one of the electrodes in a fuel cell, this platinum increases the rate of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
reduction either to water or to hydroxide
Hydroxide is a diatomic anion with chemical formula OHâ. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
or hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%â ...
.
Homogeneous catalysis
Homogeneous catalysts function in the same phase as the reactants. Typically homogeneous catalysts are dissolved in a solvent with the substrates. One example of homogeneous catalysis involves the influence of H on the esterification of carboxylic acids, such as the formation of methyl acetate fromacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
. High-volume processes requiring a homogeneous catalyst include hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
, hydrosilylation, hydrocyanation
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of pr ...
. For inorganic chemists, homogeneous catalysis is often synonymous with organometallic catalysts. Many homogeneous catalysts are however not organometallic, illustrated by the use of cobalt salts that catalyze the oxidation of p-xylene to terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced annua ...
.
Organocatalysis
Whereas transition metals sometimes attract most of the attention in the study of catalysis, small organic molecules without metals can also exhibit catalytic properties, as is apparent from the fact that manyenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s lack transition metals. Typically, organic catalysts require a higher loading (amount of catalyst per unit amount of reactant, expressed in mol%
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ex ...
amount of substance
In chemistry, the amount of substance ''n'' in a given sample of matter is defined as the quantity or number of discrete atomic-scale particles in it divided by the Avogadro constant ''N''A. The particles or entities may be molecules, atoms, ions, ...
) than transition metal(-ion)-based catalysts, but these catalysts are usually commercially available in bulk, helping to lower costs. In the early 2000s, these organocatalysts were considered "new generation" and are competitive to traditional metal
A metal (from Greek ÎŒÎÏÎ±Î»Î»ÎżÎœ ''mĂ©tallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
(-ion)-containing catalysts. Organocatalysts are supposed to operate akin to metal-free enzymes utilizing, e.g., non-covalent interactions such as hydrogen bonding. The discipline organocatalysis is divided into the application of covalent (e.g., proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the prot ...
, DMAP) and non-covalent (e.g., thiourea organocatalysis
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate ...
) organocatalysts referring to the preferred catalyst-substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
binding and interaction, respectively. The Nobel Prize in Chemistry 2021 was awarded jointly to Benjamin List and David W.C. MacMillan "for the development of asymmetric organocatalysis."
Photocatalysts
Photocatalysis is the phenomenon where the catalyst can receive light to generate anexcited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
that effect redox reactions. Singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
is usually produced by photocatalysis. Photocatalysts are components of dye-sensitized solar cell
A dye-sensitized solar cell (DSSC, DSC, DYSC or GrÀtzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a '' photoelectr ...
s.
Enzymes and biocatalysts
In biology,enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s are protein-based catalysts in metabolism
Metabolism (, from el, ΌΔÏαÎČολΟ ''metabolÄ'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
and catabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, ...
. Most biocatalysts are enzymes, but other non-protein-based classes of biomolecules also exhibit catalytic properties including ribozymes, and synthetic deoxyribozyme
Deoxyribozymes, also called DNA enzymes, DNAzymes, or catalytic DNA, are DNA oligonucleotides that are capable of performing a specific chemical reaction, often but not always catalytic. This is similar to the action of other biological enzymes, ...
s.
Biocatalysts can be thought of as an intermediate between homogeneous and heterogeneous catalysts, although strictly speaking soluble enzymes are homogeneous catalysts and membrane-bound enzymes are heterogeneous. Several factors affect the activity of enzymes (and other catalysts) including temperature, pH, the concentration of enzymes, substrate, and products. A particularly important reagent in enzymatic reactions is water, which is the product of many bond-forming reactions and a reactant in many bond-breaking processes.
In biocatalysis
Biocatalysis refers to the use of living (biological) systems or their parts to speed up (catalyze) chemical reactions. In biocatalytic processes, natural catalysts, such as enzymes, perform chemical transformations on organic compounds. Both enzy ...
, enzymes are employed to prepare many commodity chemicals including high-fructose corn syrup and acrylamide
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primary ...
.
Some monoclonal antibodies
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Monoclonal antibodies ca ...
whose binding target is a stable molecule that resembles the transition state of a chemical reaction can function as weak catalysts for that chemical reaction by lowering its activation energy. Such catalytic antibodies are sometimes called "abzymes
An abzyme (from antibody and enzyme), also called catmab (from catalytic monoclonal antibody), and most often called catalytic antibody or sometimes catab, is a monoclonal antibody with catalytic activity. Abzymes are usually raised in lab animals ...
".
Significance
 Estimates are that 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. In 2005, catalytic processes generated about $900 billion in products worldwide. The global demand for catalysts in 2014 was estimated at US$33.5 billion. Catalysis is so pervasive that subareas are not readily classified. Some areas of particular concentration are surveyed below.
Estimates are that 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. In 2005, catalytic processes generated about $900 billion in products worldwide. The global demand for catalysts in 2014 was estimated at US$33.5 billion. Catalysis is so pervasive that subareas are not readily classified. Some areas of particular concentration are surveyed below.
Energy processing
Petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
refining makes intensive use of catalysis for alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
, catalytic cracking
Fluid Catalytic Cracking (FCC) is the conversion process used in petroleum refineries to convert the high-boiling point, high-molecular weight hydrocarbon fractions of petroleum (crude oils) into gasoline, olefinic gases, and other petroleum prod ...
(breaking long-chain hydrocarbons into smaller pieces), naphtha
Naphtha ( or ) is a flammable liquid hydrocarbon mixture.
Mixtures labelled ''naphtha'' have been produced from natural gas condensates, petroleum distillates, and the distillation of coal tar and peat. In different industries and regions ''n ...
reforming and steam reforming (conversion of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
s into synthesis gas). Even the exhaust from the burning of fossil fuels is treated via catalysis: Catalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
s, typically composed of platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
and rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isoto ...
, break down some of the more harmful byproducts of automobile exhaust.
:2 CO + 2 NO â 2 CO + N
With regard to synthetic fuels, an old but still important process is the Fischer-Tropsch synthesis of hydrocarbons from synthesis gas, which itself is processed via water-gas shift reactions, catalyzed by iron. Biodiesel
Biodiesel is a form of diesel fuel derived from plants or animals and consisting of long-chain fatty acid esters. It is typically made by chemically reacting lipids such as animal fat (tallow), soybean oil, or some other vegetable oil with ...
and related biofuels require processing via both inorganic and biocatalysts.
Fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
s rely on catalysts for both the anodic and cathodic reactions.
Catalytic heaters generate flameless heat from a supply of combustible fuel.
Bulk chemicals
 Some of the largest-scale chemicals are produced via catalytic oxidation, often using
Some of the largest-scale chemicals are produced via catalytic oxidation, often using oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
. Examples include nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
(from ammonia), sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
(from sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
to sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide, also known as ''nisso sulfan'') is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an ind ...
by the contact process
The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arsenic ...
), terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced annua ...
from p-xylene, acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
from propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petrole ...
or propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used a ...
and acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular ...
from propane and ammonia.
The production of ammonia is one of the largest-scale and most energy-intensive processes. In the Haber process nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
is combined with hydrogen over an iron oxide catalyst. Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
is prepared from carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
or carbon dioxide but using copper-zinc catalysts.
Bulk polymers derived from ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
and propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petrole ...
are often prepared via Ziegler-Natta catalysis. Polyesters, polyamides, and isocyanates are derived via acid-base catalysis
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By BrĂžnstedâLowry acidâbase theory, the acid is the proton (hydrogen ion, H+) donor and the base is the proton acceptor. Typical reactions catalyze ...
.
Most carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbon ...
processes require metal catalysts, examples include the Monsanto acetic acid process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemic ...
and hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
.
Fine chemicals
Many fine chemicals are prepared via catalysis; methods include those of heavy industry as well as more specialized processes that would be prohibitively expensive on a large scale. Examples include theHeck reaction
The Heck reaction (also called the MizorokiâHeck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
, and FriedelâCrafts reaction
The FriedelâCrafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. FriedelâCrafts reactions are of two main types: alkylation reactions and acylation reactions ...
s. Because most bioactive compounds are chiral, many pharmaceuticals are produced by enantioselective catalysis (catalytic asymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
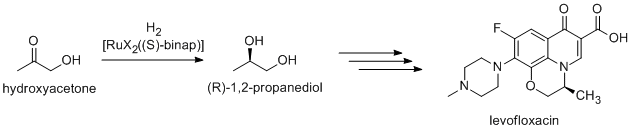
). (R)-1,2-Propandiol, the precursor to the antibacterial levofloxacin
Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori (in combination with other medications), ...
, can be synthesized efficiently from hydroxyacetone by using catalysts based on BINAP
BINAP (2,2âČ-bis(diphenylphosphino)-1,1âČ-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1â ...
-ruthenium complexes, in Noyori asymmetric hydrogenation:

Food processing
One of the most obvious applications of catalysis is thehydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
(reaction with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
gas) of fats using nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
catalyst to produce margarine
Margarine (, also , ) is a spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was orig ...
. Many other foodstuffs are prepared via biocatalysis (see below).
Environment
Catalysis affects the environment by increasing the efficiency of industrial processes, but catalysis also plays a direct role in the environment. A notable example is the catalytic role ofchlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
free radicals in the breakdown of ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
. These radicals are formed by the action of ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than ...
radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
* ''electromagnetic radiation'', such as radio waves, microwaves, infrared, visi ...
on chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and prop ...
s (CFCs).
:Cl + O â ClO + O
:ClO + O â Cl + O
History
Generally speaking, anything that increases the rate of a process is a "catalyst", a term derived from Greek ÎșαÏαλÏΔÎčÎœ, meaning "to annul," or "to untie," or "to pick up." The concept of catalysis was invented by chemist Elizabeth Fulhame and described in a 1794 book, based on her novel work in oxidation-reduction experiments. The first chemical reaction in organic chemistry that used a catalyst was studied in 1811 byGottlieb Kirchhoff
Gottlieb Sigismund Constantin Kirchhoff (19 February 1764 – 14 February 1833) was a Russian chemist of German origin. In 1792â1802, Assistant Director and then Director of the Head Pharmacy at Saint Petersburg. Corresponding member (1807â ...
who discovered the acid-catalyzed conversion of starch to glucose. The term ''catalysis'' was later used by Jöns Jakob Berzelius
Jöns is a Swedish given name and a surname.
Notable people with the given name include:
* Jöns Jacob Berzelius (1779â1848), Swedish chemist
* Jöns Budde (1435â1495), Franciscan friar from the Brigittine monastery in NaantaliVallis Gratiae ...
in 1835 to describe reactions that are accelerated by substances that remain unchanged after the reaction. Fulhame, who predated Berzelius, did work with water as opposed to metals in her reduction experiments. Other 18th century chemists who worked in catalysis were Eilhard Mitscherlich
Eilhard Mitscherlich (; 7 January 179428 August 1863) was a German chemist, who is perhaps best remembered today for his discovery of the phenomenon of crystallographic isomorphism in 1819.
Early life and work
Mitscherlich was born at Neuende ...
who referred to it as ''contact'' processes, and Johann Wolfgang Döbereiner who spoke of ''contact action. ''He developed Döbereiner's lamp
Döbereiner's lamp, also called a "tinderbox" ("Feuerzeug"), is a lighter invented in 1823 by the German chemist Johann Wolfgang Döbereiner. The lighter is based on the FĂŒrstenberger lighter (invented in Basel in 1780; in which hydrogen gas is ...
, a lighter based on hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
and a platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
sponge, which became a commercial success in the 1820s that lives on today. Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
discovered the use of platinum in catalysis. In the 1880s, Wilhelm Ostwald at Leipzig University started a systematic investigation into reactions that were catalyzed by the presence of acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s and bases, and found that chemical reactions occur at finite rates and that these rates can be used to determine the strengths of acids and bases. For this work, Ostwald was awarded the 1909 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFRâą" then "NOBEL", and on the right, the text (smaller) "NATâą" then "M ...
. Vladimir Ipatieff
Vladimir Nikolayevich Ipatieff (also Ipatyev; russian: ĐĐ»Đ°ĐŽĐžĐŒĐžŃ ĐĐžĐșĐŸĐ»Đ°Đ”ĐČĐžŃ ĐпаŃŃĐ”ĐČ); (November 21, 1867 (November 9 OS) â November 29, 1952) was a Russian and American chemist. His most important contributions are in the ...
performed some of the earliest industrial scale reactions, including the discovery and commercialization of oligomerization and the development of catalysts for hydrogenation.
Inhibitors, poisons, and promoters
An added substance that lowers the rate is called areaction inhibitor A reaction inhibitor is a substance that decreases the rate of, or prevents, a chemical reaction.
A catalyst, in contrast, is a substance that increases the rate of a chemical reaction.
Examples
* Added acetanilide slows the decomposition of drug ...
if reversible and catalyst poisons if irreversible. Promoters are substances that increase the catalytic activity, even though they are not catalysts by themselves.
Inhibitors are sometimes referred to as "negative catalysts" since they decrease the reaction rate. However the term inhibitor is preferred since they do not work by introducing a reaction path with higher activation energy; this would not lower the rate since the reaction would continue to occur by the non-catalyzed path. Instead, they act either by deactivating catalysts or by removing reaction intermediates such as free radicals.Laidler, K.J. (1978) ''Physical Chemistry with Biological Applications'', Benjamin/Cummings. pp. 415â17. .Laidler, K.J. and Meiser, J.H. (1982) ''Physical Chemistry'', Benjamin/Cummings, p. 425. . In heterogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
catalysis, coking
Coking is the heating of coal in the absence of oxygen to a temperature above 600 °C to drive off the volatile components of the raw coal, leaving a hard, strong, porous material of high carbon content called coke. Coke consists almost ent ...
inhibits the catalyst, which becomes covered by polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ic side products.
The inhibitor may modify selectivity in addition to rate. For instance, in the hydrogenation of alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbonâcarbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s to alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbonâcarbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s, a palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
(Pd) catalyst partly "poisoned" with lead(II) acetate (Pb(CHCO)) can be used. Without the deactivation of the catalyst, the alkene produced would be further hydrogenated to alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which ...
.Bender, Myron L; Komiyama, Makoto and Bergeron, Raymond J (1984) ''The Bioorganic Chemistry of Enzymatic Catalysis'' Wiley-Interscience, Hoboken, U.S.
The inhibitor can produce this effect by, e.g., selectively poisoning only certain types of active sites. Another mechanism is the modification of surface geometry. For instance, in hydrogenation operations, large planes of metal surface function as sites of hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbonâcarbon or carbonâheteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
catalysis while sites catalyzing hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
of unsaturates are smaller. Thus, a poison that covers the surface randomly will tend to lower the number of uncontaminated large planes but leave proportionally smaller sites free, thus changing the hydrogenation vs. hydrogenolysis selectivity. Many other mechanisms are also possible.
Promoters can cover up the surface to prevent the production of a mat of coke, or even actively remove such material (e.g., rhenium on platinum in platforming). They can aid the dispersion of the catalytic material or bind to reagents.
See also
References
*External links
Science Aid: Catalysts
Page for high school level science
W.A. Herrmann Technische UniversitÀt presentation
Alumite Catalyst, Kameyama-Sakurai Laboratory, Japan
Inorganic Chemistry and Catalysis Group, Utrecht University, The Netherlands
Carbons & Catalysts Group, University of Concepcion, Chile
Center for Enabling New Technologies Through Catalysis, An NSF Center for Chemical Innovation, USA
"Bubbles turn on chemical catalysts"
Science News magazine online, April 6, 2009. {{Authority control Chemical kinetics Articles containing video clips