India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the seventh-largest country by area, the second-most populous country, and the most populous democracy in the world. Bounded by the Indian Ocean on the so ...
began administration of
COVID-19 vaccine
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 ( COVID19).
Prior to the COVID19 pandemic, an e ...
s on 16 January 2021. , India has administered over 2.19 billion doses overall, including first, second and precautionary (booster) doses of the currently approved vaccines.
[(The data on this site changes daily)] In India, of the eligible population (12+) has received at least one shot, and of the eligible population (12+) is fully vaccinated.
India initially approved the
Oxford–AstraZeneca vaccine (manufactured under license by
Serum Institute of India
Serum Institute of India (SII) is an Indian biotechnology and biopharmaceuticals company, based in Pune. It is the world's largest manufacturer of vaccines. It was founded by Cyrus Poonawalla in 1966 and is a part of Cyrus Poonawalla Group.
O ...
under the trade name Covishield) and
Covaxin (a vaccine developed locally by
Bharat Biotech
Bharat Biotech International Limited (BBIL) is an Indian multinational biotechnology company headquartered in the city of Hyderabad, India engaged in the drug discovery, drug development, manufacture of vaccines, bio-therapeutics, pharmaceuti ...
). They have since been joined by the
Sputnik V
Sputnik V (russian: Спутник V, the brand name from RDIF) or Gam-COVID-Vac (russian: Гам-КОВИД-Вак, the name under which it is legally registered and produced) is an adenovirus viral vector vaccine for COVID-19 developed by t ...
(manufactured under license by
Dr. Reddy's Laboratories
Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company based in Hyderabad. The company was founded by Kallam Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy's m ...
, with additional production from Serum Institute of India being started in September),
Moderna vaccines,
Johnson & Johnson vaccine and
ZyCoV-D
ZyCoV-D is a DNA vaccine, DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is emergency use authorisation, approved ...
(a vaccine locally developed by
Zydus Cadila
Zydus Lifesciences Limited, formerly known as Cadila Healthcare Limited, is an Indian multinational pharmaceutical company headquartered in Ahmedabad, which is primarily engaged in the manufacture of generic drugs. It ranked 100th in the Fort ...
) and other vaccine candidates undergoing local clinical trials.
According to a June 2022 study published in ''
The Lancet
''The Lancet'' is a weekly peer-reviewed general medical journal and one of the oldest of its kind. It is also the world's highest-impact academic journal. It was founded in England in 1823.
The journal publishes original research articles, ...
'', COVID-19 vaccination in India prevented an additional 4.2 million deaths from December 8, 2020, to December 8, 2021.
Vaccination programme
Cumulative doses administered across the country
Total doses administered across the country as of December 2, 2022
Monthly graph of cumulative doses administered across the country
Graph of daily doses administered across the country
Graph of daily doses administered. Last updated: December 2, 2022
Vaccine administration by Gender
Vaccinations in India by Gender as of December 2, 2022
Vaccine administration by vaccine brand
Vaccinations in India by Vaccine Brand as of December 28, 2022
Vaccine administration by age group
Vaccinations in India by Age Group as of December 2, 2022
States by Vaccine Coverage
Number of Vaccination Centres
Citizens above the age of 12 can book appointments through the COWIN platform or can do a Walk-In registration on site.
All vaccine centres have registration desks, vaccine booths and observation rooms.
Vaccine certificates can be downloaded digitally through the COWIN platform, or citizens can ask for a hard copy from vaccination centres.
All government run vaccination centers provide free of cost vaccines, private centers do charge.
Background and timeline


First Phase, initial approvals, launch of vaccination programme
In September 2020, India's Health minister
Harsh Vardhan stated that the country planned to approve and begin distribution of a vaccine by the first quarter of 2021. The first recipients were to be 30 million health workers directly dealing with COVID patients.
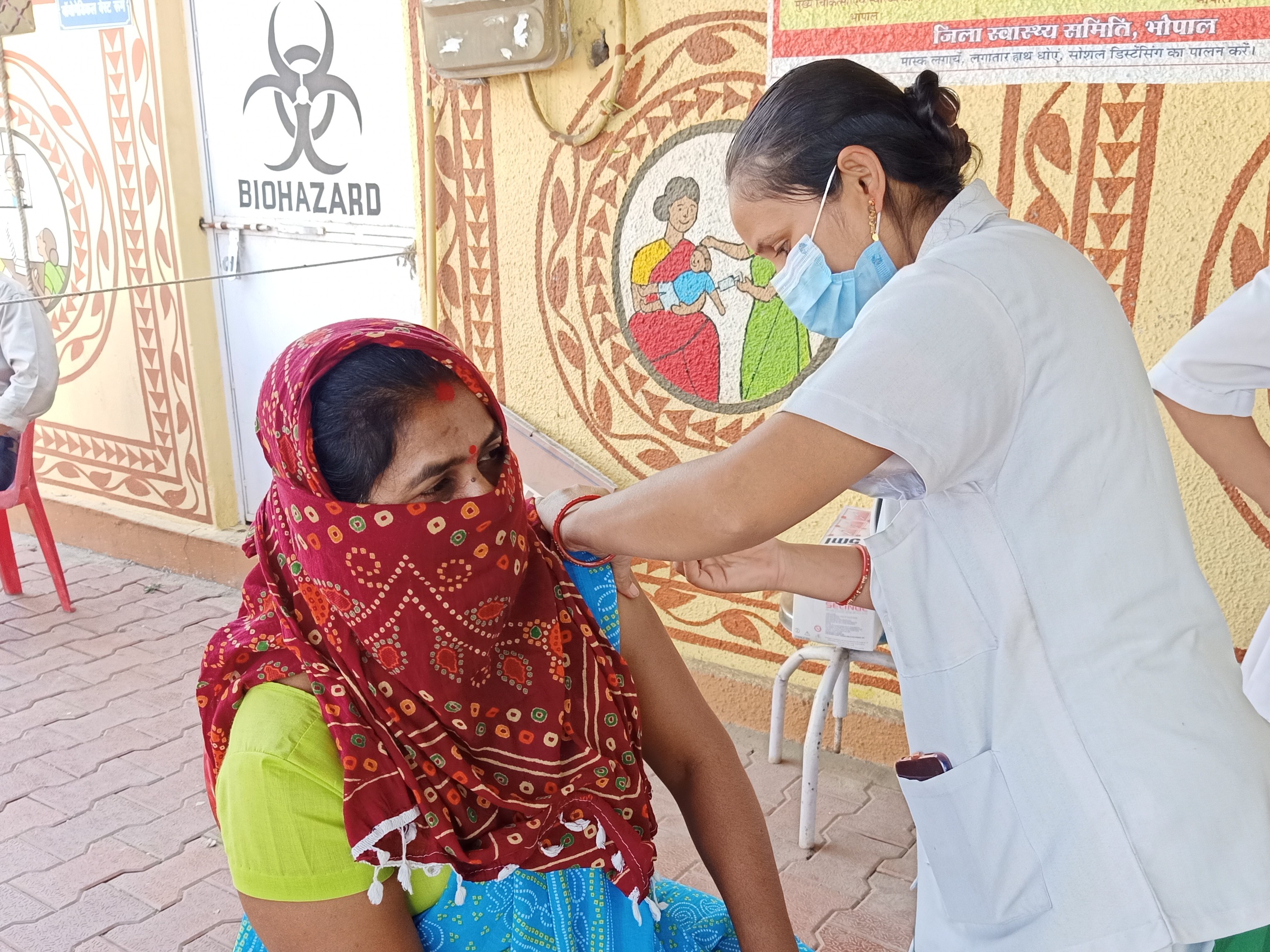
On 1 January 2021, the
Drug Controller General of India (DCGI) approved emergency use of the
Oxford–AstraZeneca vaccine (local trade name "Covishield").
On 2 January, the DCGI also granted an interim emergency use authorisation to
BBV152 (trade name "Covaxin"), a domestic vaccine developed by
Bharat Biotech
Bharat Biotech International Limited (BBIL) is an Indian multinational biotechnology company headquartered in the city of Hyderabad, India engaged in the drug discovery, drug development, manufacture of vaccines, bio-therapeutics, pharmaceuti ...
in association with the
Indian Council of Medical Research and
National Institute of Virology
The National Institute of Virology in Pune, India is an Indian virology research institute and part of the Indian Council of Medical Research (ICMR). It was previously known as 'Virus Research Centre' and was founded in collaboration with the ...
. This approval was met with some concern, as the vaccine had not then completed phase 3 clinical trials. Due to this status, those receiving Covaxin were required to sign a consent form,
while some states chose to relegate Covaxin to a "buffer stock" and primarily distribute Covishield.
India began its vaccination programme on 16 January 2021, operating 3,006 vaccination centres on the onset.
Each vaccination centre will offer either Covishield or Covaxin, but not both.
165,714 people were vaccinated on the first day of availability. Difficulties in uploading beneficiary lists at some sites caused delays.
In the first three days, 631,417 people were vaccinated. Of these, 0.18% reported side-effects and nine people (0.002%) were admitted to hospitals for observation and treatment.
Within those first days, there were concerns about low turnout, due to a combination of vaccine safety concerns, technical problems with the software used, and misinformation.
The first phase of the rollout involved health workers and frontline workers, including police, paramilitary forces, sanitation workers, and disaster management volunteers.
By 1 March, only 14 million healthcare and frontline workers had been vaccinated, falling short of the original goal of 30 million.
Second phase
The next phase of the vaccine rollout covered all residents over the age of 60, residents between the ages of 45 and 60 with one or more qualifying
comorbidities, and any health care or frontline worker that did not receive a dose during phase 1. Online registration began on 1 March via the
Aarogya Setu app and Co-WIN ("Winning over COVID-19") website. Amid the beginnings of a major second wave of infections in the country,
vaccine exports were suspended in March 2021, and the government ordered 110 million Covishield doses from SII.
[India's vaccine shortage will last months, biggest manufacturer warns](_blank)
Financial Times, 3 May 2021. The company aims to produce 100 million doses per month, but by May 2021 its production capacity was only 60–70 million doses.
CNBC, 5 May 2021. Following the conclusion of its trial, the DCGI issued a standard emergency use authorisation to Covaxin on 11 March 2021.
From 1 April, eligibility was extended to all residents over the age of 45. On 8 April, Prime Minister
Narendra Modi
Narendra Damodardas Modi (; born 17 September 1950) is an Indian politician serving as the 14th and current Prime Minister of India since 2014. Modi was the Chief Minister of Gujarat from 2001 to 2014 and is the Member of Parliament from ...
called for a four-day ''Teeka Utsav'' ("Vaccine Festival") from 11 to 14 April, with a goal to increase the pace of the program by vaccinating as many eligible residents as possible. By the end of the ''Utsav'', India had reached a total of over 111 million vaccine doses to-date.
Third phase, Sputnik V approval
On 12 April, the DCGI approved Russia's
Sputnik V
Sputnik V (russian: Спутник V, the brand name from RDIF) or Gam-COVID-Vac (russian: Гам-КОВИД-Вак, the name under which it is legally registered and produced) is an adenovirus viral vector vaccine for COVID-19 developed by t ...
vaccine for emergency use in India. A phase 3 trial was conducted in the country in September 2020, which showed 91.6% efficacy.
The local distributor
Dr. Reddy's Laboratories
Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company based in Hyderabad. The company was founded by Kallam Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy's m ...
stated that it planned to have the vaccine in India by late May 2021.
On 19 April, it was announced that the next phase of the vaccination programme would begin on 1 May, extending eligibility to all residents over the age of 18. Under phase 3, individual stakeholders were also given more flexibility in how they conduct the vaccination programme. As part of this plan, only half of the vaccines procured by the Central Drugs Laboratory from manufacturers would be distributed by the central government. This supply would go to government-run clinics and be offered free-of-charge to residents 45 and over and priority workers and siphoned off to states based on factors such as the number of active cases and how quickly they are administering vaccines. The remainder would be offered to individual states and purchased on the open market (including private hospitals), which would be able to serve residents over the age of 18.
Registration for the next phase began on 28 April; a single-day record of nearly 13.3 million people registered. Due to supply issues, several states, including Delhi, Gujarat and Madhya Pradesh announced that they would delay their wider rollouts of vaccines to later in the month.
The initial shipment of 150,000 Sputnik V doses arrived on 1 May, and began to be administered on 14 May.
An estimated 156 million doses is expected between August and December; initially, doses will be sourced from Russia, but domestic production is expected to begin by August 2021.
On 13 May, the DCGI approved phase 2 and phase 3 trials of Covaxin on children 2–18. On 14 May, health officials projected that based on the anticipated approval of additional vaccine options, it could receive at least 2.17 billion more vaccine doses from August to December 2021.
On 25 May, India exceeded 200 million vaccine doses administered in total. On 3 June, the Ministry of Health and Family Welfare pre-ordered 300 million doses of a potential fourth vaccine,
Corbevax
Corbevax is a protein subunit COVID-19 vaccine developed by Texas Children's Hospital Center for Vaccine Development and Baylor College of Medicine in Houston, Texas and Dynavax technologies based in Emeryville, California. It is licensed to ...
, which is undergoing phase 3 clinical trials.
On 23 May, the union government allowed walk-in registrations for vaccination throughout the country; a health worker at the vaccination centre would register the recipient in the Co-win vaccination database. The government claimed in an affidavit to the Supreme Court that as of June 23 about 78 per cent of vaccines had been administered via walk-in registration.
Return to centralised procurement
On 31 May, an
affidavit
An ( ; Medieval Latin for "he has declared under oath") is a written statement voluntarily made by an ''affiant'' or '' deponent'' under an oath or affirmation which is administered by a person who is authorized to do so by law. Such a statemen ...
was issued in the
Supreme Court of India
The Supreme Court of India ( IAST: ) is the supreme judicial authority of India and is the highest court of the Republic of India under the constitution. It is the most senior constitutional court, has the final decision in all legal matters ...
requesting a review of the central government's vaccine distribution strategy, suggesting that the decision to only offer doses at no charge to priority workers and residents over the age of 45 was "prima facie arbitrary and irrational".
On 7 June, Prime Minister Modi announced that India would migrate back to centralised procurement of vaccines by 21 June. In an address, Modi stated that multiple chief ministers had requested that the central government reconsider its new distribution strategy and reinstate the system it had used before May. As before, the centre will procure up to 75% of the country's vaccine supplies from manufacturers in bulk and distribute them to states at no additional charge. Vaccines would now be offered at no charge for those in the 18–44 age group. Private hospitals will still be responsible for the remaining 25% of procurement, but fees for appointments are now capped at .
On 21 June, the day these changes took effect, approximately doses were administered—India's largest single-day total until that point. The states of
Madhya Pradesh
Madhya Pradesh (, ; meaning 'central province') is a state in central India. Its capital is Bhopal, and the largest city is Indore, with Jabalpur, Ujjain, Gwalior, Sagar, and Rewa being the other major cities. Madhya Pradesh is the seco ...
and
Karnataka
Karnataka (; ISO: , , also known as Karunāḍu) is a state in the southwestern region of India. It was formed on 1 November 1956, with the passage of the States Reorganisation Act. Originally known as Mysore State , it was renamed ''Karnat ...
had the highest local totals.
Member of Parliament, Rajya Sabha
A Member of Parliament in the Rajya Sabha (abbreviated: MP) is the representative of the Indian states to the one of the two houses of the Parliament of India (Rajya Sabha). Rajya Sabha MPs are elected by the electoral college of the elected ...
P. Chidambaram
Palaniappan Chidambaram (born 16 September 1945), better known as P. Chidambaram, is an Indian politician and lawyer who currently serves as Member of Parliament, Rajya Sabha. He served as the Chairman of the Parliamentary Standing Committee ...
accused
Bharatiya Janata Party
The Bharatiya Janata Party (BJP; ; ) is a political party in India, and one of the two major Indian political parties alongside the Indian National Congress. Since 2014, it has been the ruling political party in India under Narendra Modi ...
of having "hoarded" vaccine doses in the days leading up to 21 June in order to encourage larger numbers; seven states controlled by the BJP were among the top ten states to have administered vaccine doses that day, few of these states had below-average vaccination numbers in the days leading up to 21 June (such as Madhya Pradesh, which went from 692 doses on 20 June to the next day, and numbers had dropped significantly in the state the next day).
On 23 June, India surpassed over 300 million vaccine doses administered in total. On 28 June, India overtook the
United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
in total vaccine doses administered. On 29 June, the DCGI approved the
Moderna vaccine
The Moderna COVID19 vaccine (INN: elasomeran), sold under the brand name Spikevax, is a COVID-19 vaccine developed by American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedi ...
(which is being imported by
Cipla) for emergency use.
Vinod Kumar Paul
Vinod Kumar Paul is an Indian pediatrician and physician scientist currently serving as Member, NITI Aayog. He earlier served as Professor of Neonatology at the Department of Pediatrics, All India Institute of Medical Sciences (AIIMS), New Delh ...
stated that the
Pfizer vaccine
Pfizer Inc. ( ) is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer ...
was also likely to be approved soon.
500 million mark, Johnson & Johnson Covid-19 vaccine approval and record
On 6 August 2021, India crossed the 500 million doses milestone within 6 months from the onset of the vaccination program.
On 7 August 2021, the Drug Controller General of India (DCGI) approved emergency use of the
Johnson and Johnson single-dose vaccine. On 16 Aug 2021, India administered around 8.81 million doses of COVID-19 vaccines, achieving the highest single-day record and overtaking its own previous record of 8.61 million doses, by 16 August the cumulative doses had surpassed the 55 million mark.
ZyCoV-D approval for ages 12 & above and single day vaccination records
On 20 August 2021, India granted emergency authorization to
Zydus Cadila
Zydus Lifesciences Limited, formerly known as Cadila Healthcare Limited, is an Indian multinational pharmaceutical company headquartered in Ahmedabad, which is primarily engaged in the manufacture of generic drugs. It ranked 100th in the Fort ...
's vaccine
ZyCoV-D
ZyCoV-D is a DNA vaccine, DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is emergency use authorisation, approved ...
, the world's first
DNA plasmid-based COVID-19 vaccine, for patients 12 and older.
India granted emergency use approval to the world's first
DNA based COVID-19 vaccine, manufactured by for adults and children aged 12 years and above. The vaccine is administered using a needle-free applicator. The government announced on 30 September 2021 that the ZyCoV-D vaccine will be a three dose vaccine and it will be included in the Covid vaccination programme of India.
Since August 10, 2021, foreign nationals residing in India can now receive the COVID-19 vaccine by registering themselves on the Cowin platform; like other eligible beneficiaries, the foreign nationals can book a slot via the portal and use their passport as a document to verify their identity for the registration process.
By 26 August 2021, 50% of the adult population in India were inoculated with at least one dose of the approved vaccines, which included 99% coverage among
healthcare workers and 100% front-line workers for the first dose.
On 27 August 2021, India crossed the milestone of administering more than 10 million (1 crore) doses of COVID-19 vaccine in a single day, setting a new world record.
Uttar Pradesh
Uttar Pradesh (; , 'Northern Province') is a state in northern India. With over 200 million inhabitants, it is the most populated state in India as well as the most populous country subdivision in the world. It was established in 1950 ...
topped the list with 2.8 million doses, followed by
Karnataka
Karnataka (; ISO: , , also known as Karunāḍu) is a state in the southwestern region of India. It was formed on 1 November 1956, with the passage of the States Reorganisation Act. Originally known as Mysore State , it was renamed ''Karnat ...
with 1 million doses and
Maharashtra
Maharashtra (; , abbr. MH or Maha) is a states and union territories of India, state in the western India, western peninsular region of India occupying a substantial portion of the Deccan Plateau. Maharashtra is the List of states and union te ...
at third with 0.98 million doses.
On 29 August 2021,
Himachal Pradesh
Himachal Pradesh (; ; "Snow-laden Mountain Province") is a state in the northern part of India. Situated in the Western Himalayas, it is one of the thirteen mountain states and is characterized by an extreme landscape featuring several peaks ...
became the first state to complete administering first doses of COVID-19 vaccines to 100% of the population. On 31 August, India again set another single-day vaccination record by inoculating around 12 million (1.2 Crore) doses in 24 hours.
By September 2021, all adult people in Sikkim, Dadra and Nagar Haveli, Himachal Pradesh, Goa, Ladakh, and Lakshadweep have received at least one dose of Covid vaccine as the cumulative jabs administered in the country crossed 75 crores. Many city corporations, talukas, gram panchayats and districts had also administered the first dose of COVID-19 vaccination to 100 per cent of their adult population.
As many as 25 million people were vaccinated on Narendra Modi's birthday on 17 September 2021. This is the highest single-day vaccination tally so far in the whole world. There are allegations that a lot of pressure could have been put on officials to increase and twist to record vaccination numbers on the Prime Minister's birthday as some people who had not received their Covid shots somehow seem to have been issued vaccination certificates including those who are deceased.
On 27 September, India administered over 1 crore vaccine doses for the fifth time, and total vaccination coverage crossed 86 crore (860 million). On 2 October, this increased to 960 million.
Booster doses and pediatric vaccines
By September 2021, several companies had received approval to begin clinical trials on COVID-19 vaccines for children. Discussions were also emerging over whether India would utilize vaccine booster doses, with the government having maintained that they were studying their applicability, and that they were focused on their goal to administer a primary series of vaccine doses to all adults. On 21 October 2021, India crossed the one billion mark for administered doses.
On 26 December 2021, Prime Minister Modi announced that vaccine eligibility would be extended to youth 15 to 18 years of age from 3 January 2022 (with online registration opened on 1 January 2022); this age group would exclusively receive Covaxin. It was also announced that booster doses would become available from 10 January 2022, beginning with frontline workers, and residents over the age of 60 with comorbidities. Within this cohort, doses would be prioritized to those who had received their second dose at least nine months prior.
On 14 March 2022, Union health minister
Mansukh Mandaviya
Mansukh Laxmanbhai Mandaviya (born 1 June 1972) is an Indian politician currently serving as the Minister of Health and Family Welfare and Chemicals and Fertilizers of India. He is also a Rajya Sabha member from Gujarat.
Early life
Mans ...
announced that vaccine eligibility would be extended to children 12–14, and that this group would exclusively receive Corbevax. Booster eligibility was also extended to all adults 60 and older. On 8 April 2022, the
Ministry of Health and Family Welfare announced that all adults 18 and over would become eligible for booster doses beginning 10 April, and that they would be available via private vaccination centres.
In May 2022, the
Supreme Court of India
The Supreme Court of India ( IAST: ) is the supreme judicial authority of India and is the highest court of the Republic of India under the constitution. It is the most senior constitutional court, has the final decision in all legal matters ...
ruled that COVID-19 vaccine mandates were unconstitutional, citing the "emerging scientific opinion" that COVID-19 vaccines did not reduce the risk of transmission by those who are vaccinated.
Vaccine development and distribution
As of early May 2020, there were over 30 vaccine candidates in development in India, many of which were already in pre-clinical trials.
The Pune-based
Serum Institute of India
Serum Institute of India (SII) is an Indian biotechnology and biopharmaceuticals company, based in Pune. It is the world's largest manufacturer of vaccines. It was founded by Cyrus Poonawalla in 1966 and is a part of Cyrus Poonawalla Group.
O ...
(SII) is the world's largest vaccine maker. This existing capacity enabled India to be a major participant in the
COVAX programme to distribute vaccines to developing countries. In February 2020, SII had begun animal trials of vaccine candidates. SII announced in April 2020 that it would apply for clinical trials from the
Drug Controller General of India (DCGI) in April 2020. SII president
Adar Poonawalla said that a vaccine would be delivered within a year, but projected an efficacy between 70 and 80%.
In August 2020, SII received approvals for phase 2 and phase 3 trials of its version of
a vaccine being developed by
AstraZeneca and the
University of Oxford
, mottoeng = The Lord is my light
, established =
, endowment = £6.1 billion (including colleges) (2019)
, budget = £2.145 billion (2019–20)
, chancellor ...
's
Vaccitech
Vaccitech plc is a biotechnology company developing vaccines and immunotherapies for infectious diseases and cancer, such as hepatitis B, HPV and prostate cancer.
Technology
The company's platform includes Chimpanzee Adenovirus Oxford ( ChAdOx) a ...
. SII joined
GAVI
GAVI, officially Gavi, the Vaccine Alliance (previously the GAVI Alliance, and before that the Global Alliance for Vaccines and Immunization) is a public–private global health partnership with the goal of increasing access to immunization ...
in a partnership with the
Bill & Melinda Gates Foundation
The Bill & Melinda Gates Foundation (BMGF), a merging of the William H. Gates Foundation and the Gates Learning Foundation, is an American private foundation founded by Bill Gates and Melinda French Gates. Based in Seattle, Washington, it was l ...
to produce 100 million doses of vaccine for developing countries. The SII planned to manufacture 1.5 and 2.5 billion doses of the AstraZeneca vaccine per-year under the trade name "Covishield".
By its approval in January 2021, the company had stockpiled 50 million doses, but well short of its own target of 400 million.
[Adar Poonawalla: 'Aggression over jabs is overwhelming Everyone expects to get theirs first](_blank)
''The Times'', 1 May 2021. The government ordered 21 million doses to be delivered by February, but the company said no indication of any further orders were given.
The company began to export the remaining stocks instead.
Hyderabad-based
Bharat Biotech
Bharat Biotech International Limited (BBIL) is an Indian multinational biotechnology company headquartered in the city of Hyderabad, India engaged in the drug discovery, drug development, manufacture of vaccines, bio-therapeutics, pharmaceuti ...
, in collaboration with U.S.-based FluGen, expected to begin the first clinical trials of a nasal vaccine by late-2020. The
Indian Council of Medical Research partnered with Bharat Biotech in May 2020 to develop a COVID vaccine entirely within India. In June 2020, it received DCGI approval to begin phase 1 and phase 2 trials on its vaccine,
BBV152 (trade name "Covaxin"). In September 2020, it was reported that in pre-clinical trials on animals, Covaxin was able to build immunity. In July 2021, Bharat Biotech reported the vaccine to be effective against asymptomatic cases, effective against symptomatic cases, effective against severe COVID-19 infection, and effective against the
Delta variant.
On 20 April 2021, Bharat Biotech announced that it had expanded its production capabilities for Covaxin to 700 million doses per-year.
Cadila Healthcare
Zydus Lifesciences Limited, formerly known as Cadila Healthcare Limited, is an Indian multinational pharmaceutical company headquartered in Ahmedabad, which is primarily engaged in the manufacture of generic drugs. It ranked 100th in the Fortu ...
began vaccine development in March 2020, including a
viral vector vaccine
A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material ( DNA), which can be transcribed by the recipient's host cells as mRNA coding for a desired protein (or: antigen) to elicit an immune response. , six viral v ...
and a
DNA plasmid vaccine. In mid-July 2020, Cadila held early human trials of its vaccine candidate
ZyCoV-D
ZyCoV-D is a DNA vaccine, DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is emergency use authorisation, approved ...
, and received approval for phase 3 trials in January 2021. It began large-scale production in April 2021, with Cadila expecting to receive emergency authorisation between May and June 2021. On 1 July 2021, Cadila Healthcare reported the efficacy to be 66.6% against symptomatic COVID-19 and 100% against moderate or severe disease in its interim analysis of its phase 3 trial data.
In September 2020,
Dr. Reddy's partnered with the
Russian Direct Investment Fund (RDIF) to conduct phase 3 trials of the
Sputnik V vaccine
Sputnik V (russian: Спутник V, the brand name from RDIF) or Gam-COVID-Vac (russian: Гам-КОВИД-Вак, the name under which it is legally registered and produced) is an adenovirus viral vector vaccine for COVID-19 developed by t ...
in India, and to distribute the vaccine there once approved. In April 2021, RDIF CEO
Kirill Dmitriev
Kirill Alexandrovich Dmitriev (russian: Кирилл Александрович Дмитриев; born 12 April 1975) is the CEO of the Russian Direct Investment Fund (RDIF), a $10 billion sovereign wealth fund created by the Russian government ...
told
NDTV
New Delhi Television Ltd is an Indian news media company focusing on broadcast and digital news publication. The company is considered to be a legacy brand that pioneered independent news broadcasting in India, and is credited for launching t ...
that they had "five great manufacturers in India" who would be producing the vaccine, and felt that the country could become Sputnik V's "production hub" for use and export. Dr. Reddy's is also working with the RDIF on approval of "
Sputnik Light
Sputnik Light (russian: Спутник Лайт, Sputnik Layt or Lajt) is a single dose COVID-19 vaccine developed by the Gamaleya Research Institute of Epidemiology and Microbiology. It consists of the first dose of the Sputnik V vaccine, wh ...
"—a regiment of Sputnik V consisting only of the first dose.
In April 2021, phase 3 clinical trials were approved for another vaccine,
Corbevax
Corbevax is a protein subunit COVID-19 vaccine developed by Texas Children's Hospital Center for Vaccine Development and Baylor College of Medicine in Houston, Texas and Dynavax technologies based in Emeryville, California. It is licensed to ...
, a protein subunit that is being developed by
BioE, the
Baylor College of Medicine
Baylor College of Medicine (BCM) is a medical school and research center in Houston, Texas, within the Texas Medical Center, the world's largest medical center. BCM is composed of four academic components: the School of Medicine, the Graduate Sc ...
, and Dynavax Technologies.
On 2 June 2021, the DCGI removed the requirement that India-specific clinical trials (bridging trials) be held for vaccine candidates developed outside of India, provided that they are already approved by a recognised international public health agency such as the
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
(WHO),
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or Euro ...
(EMA), US
Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respon ...
(FDA), UK
Medicines and Healthcare products Regulatory Agency
The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably ...
(MHRA), or Japan's
Pharmaceuticals and Medical Devices Agency
The (PhMDA) is an Independent Administrative Institution responsible for ensuring the safety, efficacy and quality of pharmaceuticals and medical devices in Japan. It is similar in function to the Food and Drug Administration in the United State ...
. These changes were intended to help expedite the availability of vaccines already in use in other countries.
In mid-July, it was reported that approval of the Moderna and Pfizer vaccines, as well as a shipment of vaccines donated by the United States (which includes the AstraZeneca,
Janssen, Moderna, and Pfizer vaccines), had faced delays due to requests from their manufacturers for
indemnity
In contract law, an indemnity is a contractual obligation of one party (the ''indemnitor'') to compensate the loss incurred by another party (the ''indemnitee'') due to the relevant acts of the indemnitor or any other party. The duty to indemni ...
clauses from Indian authorities, which would relieve them from legal liability for adverse reactions.
On 21 September 2021, the Indian Government said that it will not purchase the Pfizer-BioNTech and Moderna vaccines as domestic output of more affordable and easier-to-store vaccines has jumped.
In the
Quad summit, Prime Minister Narendra Modi announced that India would make 8 million doses of
J&J vaccine available by the end of October under the Quad vaccine partnership. It will be manufactured in India by the Biological E. This would be ready by the end of October, compatible with our decision to resume vaccine export.
On 6 February 2022, DCGI approved Sputnik Light Covid vaccine for emergency use in India.
Vaccine deployment strategies
Optimizing vaccination deployment for India includes tailoring of strategies to the demographic and epidemiologic situation by modeling vaccination scenarios, explore measures to reach children at risk and co-optimizing vaccination strategy for societal and individual benefit in parallel.
Vaccine on order
In September 2021, India's government announced that it would not buy COVID-19 shots from Pfizer (PFE.N)/BioNTech (22UAy.DE) and Moderna (MRNA.O), mainly because the domestic output of more affordable and easier-to-store vaccines of Serum Institute of India, Bharat Biotech & Cadila Healthcare has jumped.
India will make 8 million doses of the Johnson and Johnson vaccine available by the end of October under the Quad vaccine partnership.
Vaccines in trial stage
Global distribution of India-manufactured vaccines
Vaccine Maitri (''English:'' Vaccine Friendship) is a
humanitarian
Humanitarianism is an active belief in the value of human life, whereby humans practice benevolent treatment and provide assistance to other humans to reduce suffering and improve the conditions of humanity for moral, altruistic, and emotional ...
initiative undertaken by the
Indian government
The Government of India (ISO 15919, ISO: ; often abbreviated as GoI), known as the Union Government or Central Government but often simply as the Centre, is the Government, national government of the Republic of India, a federal democracy lo ...
to provide
COVID-19 vaccine
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 ( COVID19).
Prior to the COVID19 pandemic, an e ...
s to countries around the world.
The government started providing vaccines from 20 January 2021. As of 6March 2022, India had delivered around 16.3 cror doses of vaccines to 96 countries.
Of these, 1.43 cror doses were gifted to 46 countries by the
Government of India
The Government of India (ISO: ; often abbreviated as GoI), known as the Union Government or Central Government but often simply as the Centre, is the national government of the Republic of India, a federal democracy located in South Asia, c ...
. The remaining 10.71 cror were supplied by the vaccine producer under its commercial and, 4.15 cror doses were supplied throw
COVAX obligations. In late March 2021, the
Government of India
The Government of India (ISO: ; often abbreviated as GoI), known as the Union Government or Central Government but often simply as the Centre, is the national government of the Republic of India, a federal democracy located in South Asia, c ...
temporarily froze exports of the
Covishield, citing
India's own COVID crisis and the domestic need for these vaccines.
The Health Minister of India,
Mr. Mansukh Mandaviya announced in September that India will resume the export of vaccines from October to the rest of the world.


200,000 doses of COVID-19 vaccines were gifted by India to the
UN peacekeepers on 27 March to be distributed to all peacekeeping missions.
Vaccines
India has two approved COVID-19 vaccines:
Covishield and
Covaxin. Both of them were exported and used in foreign grants by the Government of India.
Covishield
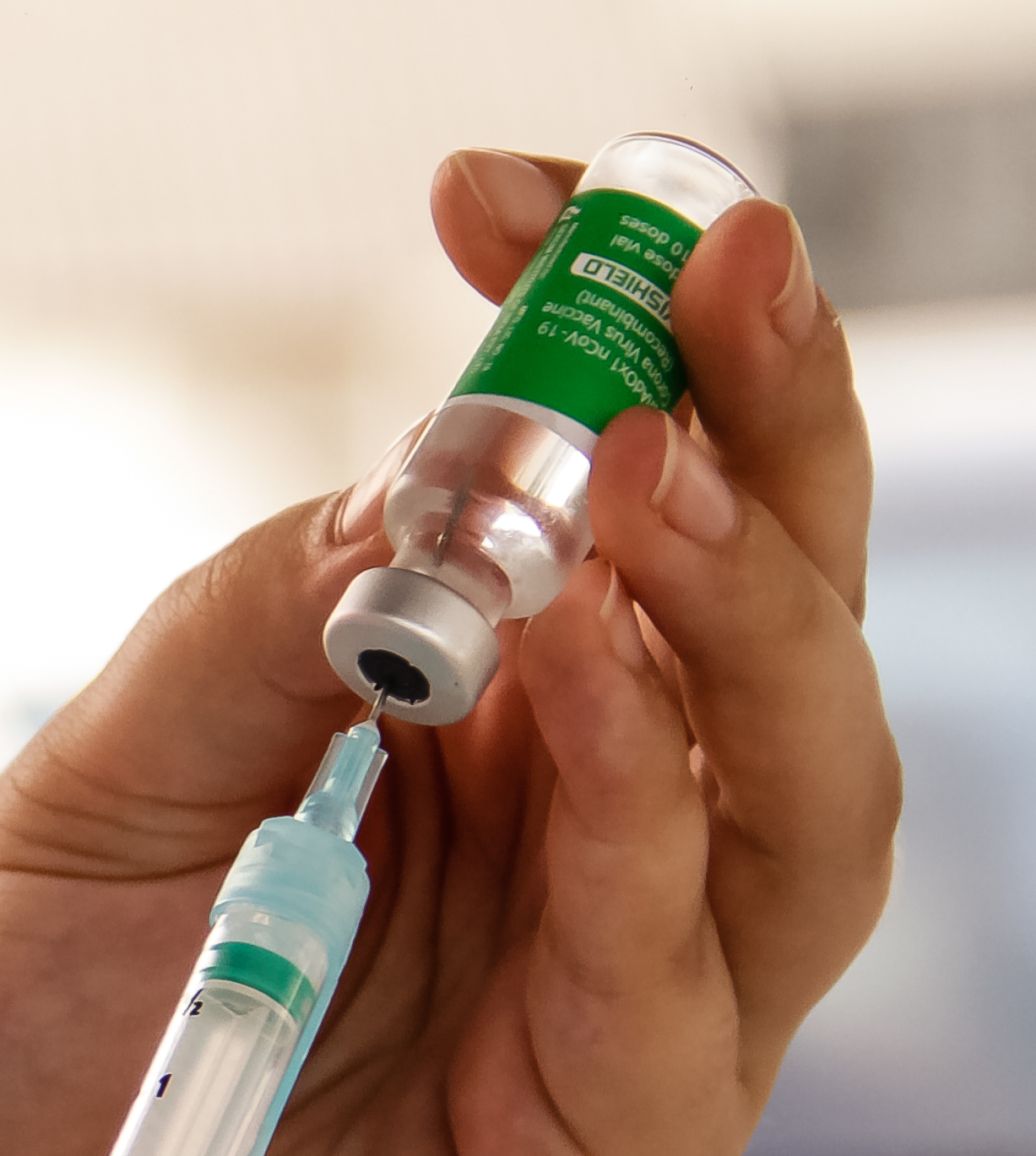
On 1 January 2021, the
Drug Controller General of India, approved the emergency or conditional use of
Covishield. Covishield is developed by the
University of Oxford
, mottoeng = The Lord is my light
, established =
, endowment = £6.1 billion (including colleges) (2019)
, budget = £2.145 billion (2019–20)
, chancellor ...
and its spin-out company,
Vaccitech
Vaccitech plc is a biotechnology company developing vaccines and immunotherapies for infectious diseases and cancer, such as hepatitis B, HPV and prostate cancer.
Technology
The company's platform includes Chimpanzee Adenovirus Oxford ( ChAdOx) a ...
.
Covaxin

On 2 January 2021,
Covaxin India's first COVID-19 vaccine, developed by
Bharat Biotech
Bharat Biotech International Limited (BBIL) is an Indian multinational biotechnology company headquartered in the city of Hyderabad, India engaged in the drug discovery, drug development, manufacture of vaccines, bio-therapeutics, pharmaceuti ...
in association with the
Indian Council of Medical Research and
National Institute of Virology
The National Institute of Virology in Pune, India is an Indian virology research institute and part of the Indian Council of Medical Research (ICMR). It was previously known as 'Virus Research Centre' and was founded in collaboration with the ...
received approval from the Drug Controller General of India for its emergency or conditional usage.
Vaccine supply
India kicked off international shipment of the vaccines on 20 January 2021, only four days after starting its own vaccination program.
Bhutan and Maldives were the first countries to receive vaccines as a grant by India. This was quickly followed by shipments to Nepal, Bangladesh, Myanmar and Seychelles.
By mid-March 2021, India was also supplying vaccines on a commercial basis to countries including Canada, the UK, and Saudi Arabia.
As of 21 February 2022, India had exported total 16,29,63,500 doses including 1,42,67,000 vaccine provided as grant to more than 96 nations and, UN Peacekeepers and UN Health workers.
The
Serum Institute of India
Serum Institute of India (SII) is an Indian biotechnology and biopharmaceuticals company, based in Pune. It is the world's largest manufacturer of vaccines. It was founded by Cyrus Poonawalla in 1966 and is a part of Cyrus Poonawalla Group.
O ...
was selected as a key supplier of cost-effective COVID-19 vaccines to the
COVAX initiative, 19.8 million doses of
Covishield vaccines were supplied by India to various countries through the initiative.
In May, when COVAX was already short 140 million doses, the Serum Institute announced that it expected to maintain its suspension of vaccine deliveries to COVAX through the end of 2021 due to the second wave of COVID-19 in India and the US ban on export of key raw materials.
International reaction
International organizations
*
IMF
The International Monetary Fund (IMF) is a major financial agency of the United Nations, and an international financial institution, headquartered in Washington, D.C., consisting of 190 countries. Its stated mission is "working to foster globa ...
: IMF chief economist
Gita Gopinath
Gita Gopinath (born 8 December 1971) is an Indian-American economist who has served as the first deputy managing director of the International Monetary Fund (IMF), since 21 January 2022. She had previously served as chief economist of the IMF be ...
lauded India for playing a key role during the crisis by dispatching vaccines to many countries. She said "I also want to mention that India really stands out in terms of its vaccine policy. If you look at where exactly is one manufacturing hub for vaccines in the worldthat will be India."
Countries
*
of the
OACPS has thanked Indian efforts in delivering vaccines to developing and least developed countries.
* Prime minister
Khadga Prasad Oli
Khadga Prasad Sharma Oli ( ne, खड्गप्रसाद शर्मा ओली, ; born 22 February 1952) is a Nepalese politician and former Prime Minister of Nepal. He served three terms as prime minister from 11 October 2015 to 3 Augu ...
thanked India stating; "We got an early chance to administer the Covid-19 vaccine. For this, I thank our neighbouring nation
India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the seventh-largest country by area, the second-most populous country, and the most populous democracy in the world. Bounded by the Indian Ocean on the so ...
, its government, the people, and especially Prime Minister
Narendra Modi
Narendra Damodardas Modi (; born 17 September 1950) is an Indian politician serving as the 14th and current Prime Minister of India since 2014. Modi was the Chief Minister of Gujarat from 2001 to 2014 and is the Member of Parliament from ...
. They sent 10 lakh doses of vaccines to us as a grant within a week of the roll-out in India."
* on behalf of
CARICOM thanked India for providing vaccine supplies to them.
* Prime Minister
Mia Amor Mottley
Mia Amor Mottley, (born 1 October 1965) is a Barbadians, Barbadian politician and attorney who has served as the eighth prime minister of Barbados since 2018 and as Leader of the Barbados Labour Party (BLP) since 2008. Mottley is the first wom ...
thanked Prime Minister
Narendra Modi
Narendra Damodardas Modi (; born 17 September 1950) is an Indian politician serving as the 14th and current Prime Minister of India since 2014. Modi was the Chief Minister of Gujarat from 2001 to 2014 and is the Member of Parliament from ...
for the supply of "Made in India" COVID-19 vaccines. She tweeted, "PM Modi made it possible for more than 40,000 persons in Barbados and tens of thousands elsewhere, to receive their 1st dose of COVISHIELD via Vaccine Maitri before receiving his. A genuine demonstration of generosity. Thank you and we wish you continued good health."
* Prime Minister
Gaston Browne had thanked Prime Minister of India
Narendra Modi
Narendra Damodardas Modi (; born 17 September 1950) is an Indian politician serving as the 14th and current Prime Minister of India since 2014. Modi was the Chief Minister of Gujarat from 2001 to 2014 and is the Member of Parliament from ...
"for demonstrating an act of benevolence, kindness and empathy", for sending vaccines to Caribbean countries.
* Afghanistan's ambassador to India Farid Mamundzay said "Thank you, India for providing Afghan people lifesaving gift on the first day of 2022!"
Leaders who received vaccines provided by India
* –
Heng Samrin
Heng Samrin ( km, ហេង សំរិន; born 25 May 1934) is a Cambodian politician who serves as the President of the National Assembly of Cambodia. Between 1979 and 1992, he was the ''de facto'' leader of the Hanoi-backed People's Republi ...
,
Say Chhum
* –
KP Sharma Oli
Khadga Prasad Sharma Oli ( ne, खड्गप्रसाद शर्मा ओली, ; born 22 February 1952) is a Nepalese politician and former Prime Minister of Nepal. He served three terms as prime minister from 11 October 2015 to 3 Augu ...
Gallery
COVID-19 vaccine from India to Seychelles.jpg, COVID-19 vaccine from India arrives at Seychelles
Seychelles (, ; ), officially the Republic of Seychelles (french: link=no, République des Seychelles; Creole: ''La Repiblik Sesel''), is an archipelagic state consisting of 115 islands in the Indian Ocean. Its capital and largest city, V ...
.
File:Delivery of Covishield COVID-19 vaccine from India to Brazil (2021) A.jpg, Delivery of Covishield COVID-19 vaccine from India to Brazil
Brazil ( pt, Brasil; ), officially the Federative Republic of Brazil (Portuguese: ), is the largest country in both South America and Latin America. At and with over 217 million people, Brazil is the world's fifth-largest country by area ...
.
File:Delivery of Covishield COVID-19 vaccine from India to Brazil (2021) D.jpg, Delivery of Covishield COVID-19 vaccine from India to Brazil.
File:Vaccination drive for COVID prevention in Bhopal, India 02.jpg, Vaccination drive for COVID prevention in Bhopal, India.
Vaccination rollout statistics by State or UT
As of December 2, 2022, populations above the age of 12 are only eligible for vaccination.
The percentage of population inoculated shown in the table above is according to the 2021 projected population of India with all age groups.
According to the government, based on the projected mid-year count for 2021, the country's total population aged 12 years and above is approximately 108 crore. Around 94.59% of the 12+ population of the country have received at least one dose of the COVID-19 vaccine since the vaccination drive began.
In addition to this, 87.71% of the eligible population has been fully vaccinated.
Vaccine acceptance in India
Over 80% of the population of India have a positive response for getting anti covid shots. India has one of the lowest vaccine hesitancy in the world.
There was vaccine hesitancy in the initial months of 2021, especially in rural India and among poor and tribal populations. Constant government and public awareness drastically reduced vaccine hesitancy. Since May 2021, more than half of daily doses administered in India have been from rural parts. Vaccine centers in India have witnessed large number of people willing to get covid vaccine resulting in overcrowding and mismanagement. Many centers across India in months of April & May reported severe shortage of covid vaccines due to large crowds turning up for vaccination. In cities like Mumbai, New Delhi, Bengaluru many people even after waiting for hours did not receive their covid vaccine due to shortage. Since July, vaccine supply has drastically increased thus India is vaccinating at a very fast pace.
One study published on vaccine acceptance shows that 79.5% of people from Delhi want to take a COVID-19 vaccine. In another study which was published from West Bengal, a state in Eastern India, has shown that 77.27% of people want to take the COVID-19 vaccine. According to the finding from these two studies, it can be expected that over 75% of people want to get a COVID-19 vaccine.
Adverse events
Like many other vaccinations, COVID-19 vaccines also have a risk of causing side effects. According to India's Union Ministry of Health and Family Welfare, the most common side-effects include pain or swelling at the injection site, fever, irritability and headaches. The
UK Government also lists fatigue, nausea and joint pain as common side-effects of the Oxford vaccine (known as Covishield in India). Medical experts maintain that vaccines used are safe and their benefits outweigh the risks. It is also important to note that adverse cases do not necessarily have a causal relationship with the vaccines.
A total of 617 serious adverse events were reported until March 29. Of these, 180 cases resulted in death. The Immunisation Technical Support Unit at the federal health ministry examined 192,000 case reports, including 12,400 deaths. In more than half of the examined cases of death, the cause of death was found to be
acute coronary syndrome
Acute coronary syndrome (ACS) is a syndrome (a set of signs and symptoms) due to decreased blood flow in the coronary arteries such that part of the heart muscle is unable to function properly or dies. The most common symptom is centrally loca ...
. However, the documentation had been completed for only 3,500 cases.
By 7 June, 26,000 adverse events had been reported following immunisation. Of this, 24,901 were minor, 412 were significant and 887 were serious. 488 deaths were also reported, including 301 men and 178 women (details of 9 deaths were not available). Both vaccines had an adverse reaction rate of about 0.01% and a fatality rate of around 0.0001% - 24,703 events and 457 deaths from 210 million Covishield doses, 1,497 events and 20 deaths following 25 million Covaxin doses. Maharashtra reported the most adverse events (4,521), followed by Kerala (4,074), Karnataka (2,650) and West Bengal (1,456).
On 15 June, the government published a review of case reports that had occurred between 5 February and 31 March 2021, focusing on 31 cases and one death from
anaphylaxis
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the follow ...
that were believed to have been attributed to the vaccine, out of nearly 60 million doses administered in the time period. Only three of these cases, and the single death of a 68-year-old patient, were determined to be "vaccine-product related", with the remainder having been classified as coincidental, indeterminate, or unclassifiable. The report stated that "mere reporting of deaths and hospitalisations as serious adverse events does not automatically imply that the events were caused due to vaccines. Only properly conducted investigations and causality assessments can help in understanding if any causal relationship exists between the event and the vaccine."
References
Footnote references
Web References
Notes
External links
*
*
*
*
*
{{Vaccines
India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the seventh-largest country by area, the second-most populous country, and the most populous democracy in the world. Bounded by the Indian Ocean on the so ...
vaccination
Vaccination is the administration of a vaccine to help the immune system develop immunity from a disease. Vaccines contain a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism. In stimulating ...
2020 in India
2021 in India


 On 1 January 2021, the Drug Controller General of India (DCGI) approved emergency use of the Oxford–AstraZeneca vaccine (local trade name "Covishield"). On 2 January, the DCGI also granted an interim emergency use authorisation to BBV152 (trade name "Covaxin"), a domestic vaccine developed by
On 1 January 2021, the Drug Controller General of India (DCGI) approved emergency use of the Oxford–AstraZeneca vaccine (local trade name "Covishield"). On 2 January, the DCGI also granted an interim emergency use authorisation to BBV152 (trade name "Covaxin"), a domestic vaccine developed by 
 On 1 January 2021, the Drug Controller General of India, approved the emergency or conditional use of Covishield. Covishield is developed by the
On 1 January 2021, the Drug Controller General of India, approved the emergency or conditional use of Covishield. Covishield is developed by the  On 2 January 2021, Covaxin India's first COVID-19 vaccine, developed by
On 2 January 2021, Covaxin India's first COVID-19 vaccine, developed by