CNBr Activated Matrices Reaction on:
[Wikipedia]
[Google]
[Amazon]
Cyanogen bromide is the
2 NaCN + Br2 -> (CN)2 + 2 NaBr
:(CN)2 + Br2 -> 2 (CN)Br
When refrigerated the material has an extended shelflife. Like some other cyanogen compounds, cyanogen bromide undergoes an exothermic trimerisation to 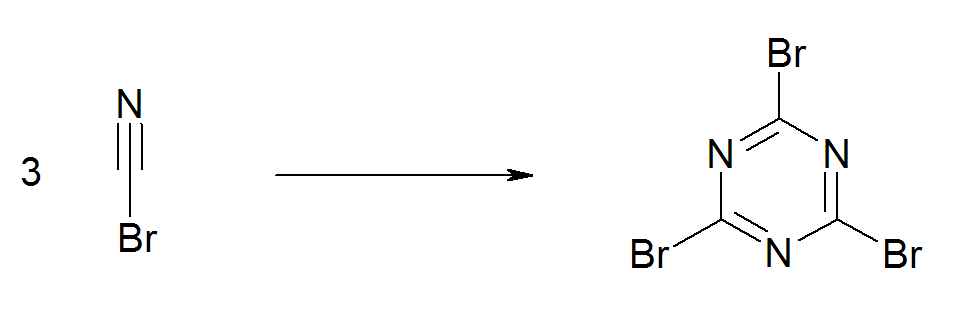 Cyanogen bromide is
Cyanogen bromide is (CN)Br + H2O -> HCN + HOBr
 The electron density in cyanogen bromide is shifted away from the carbon atom, making it unusually
The electron density in cyanogen bromide is shifted away from the carbon atom, making it unusually
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
(CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, cl ...
s, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. The compound is classified as a pseudohalogen.
Synthesis, basic properties, and structure
The carbon atom in cyanogen bromide is bonded to bromine by a single bond and to nitrogen by a triple bond (i.e. ). The compound is linear and polar, but it does not spontaneously ionize in water. It dissolves in both water and polar organic solvents. Cyanogen bromide can be prepared by oxidation of sodium cyanide with bromine, which proceeds in two steps via the intermediate cyanogen (): :cyanuric bromide
Cyanuric bromide is a heterocyclic compound with formula C3N3Br3. It contains a six-membered ring of alternating nitrogen and carbon atoms, with a bromine atom attached to each carbon. It is formed by the spontaneous trimerisation of cyanogen brom ...
(). This reaction is catalyzed by traces of bromine, metal salts, acids and bases. For this reason, experimentalists avoid brownish samples.
:hydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
to release hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
and hypobromous acid
:Biochemical applications
The main uses of cyanogen bromide are to immobilize proteins, fragment proteins by cleavingpeptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
s, and synthesize cyanamides and other molecules.
Protein immobilization
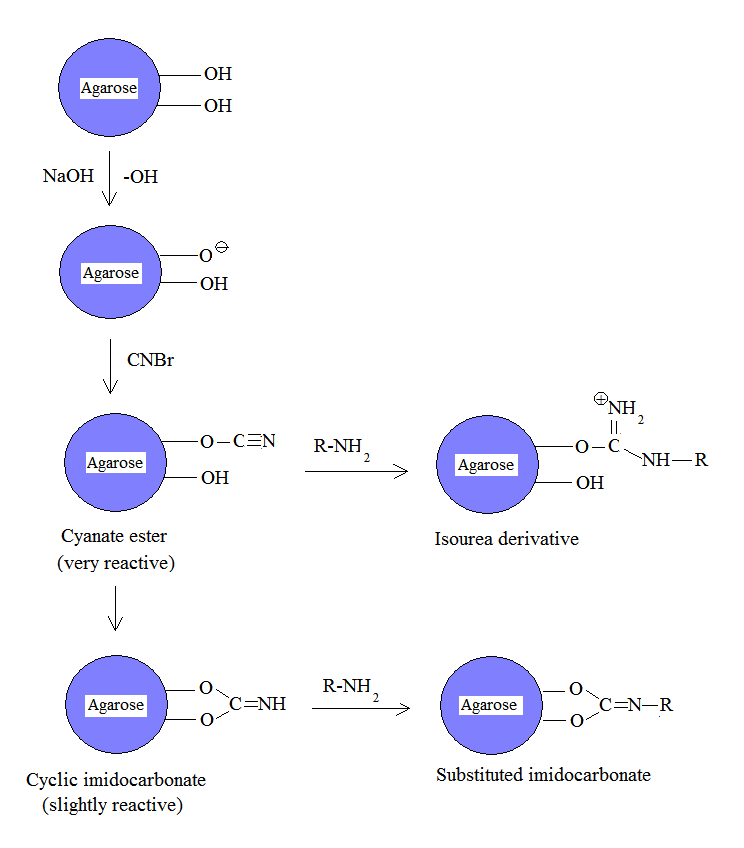
Cyanogen bromide is often used to immobilize proteins by coupling them toreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s such as agarose for affinity chromatography. Because of its simplicity and mild pH conditions, cyanogen bromide activation is the most common method for preparing affinity gels. Cyanogen bromide is also often used because it reacts with the hydroxyl groups on agarose to form cyanate esters and imidocarbonates. These groups are reacted with primary amines in order to couple the protein onto the agarose matrix, as shown in the figure. Because cyanate esters are more reactive than are cyclic imidocarbonates, the amine will react mostly with the ester, yielding isourea derivatives, and partially with the less reactive imidocarbonate, yielding substituted imidocarbonates.
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a ligand to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak anion exchangers.
Protein cleavage
Cyanogen bromide hydrolyzespeptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
s at the C-terminus of methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ro ...
residues. This reaction is used to reduce the size of polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
segments for identification and sequencing
In genetics and biochemistry, sequencing means to determine the primary structure (sometimes incorrectly called the primary sequence) of an unbranched biopolymer. Sequencing results in a symbolic linear depiction known as a sequence which succ ...
.
Mechanism
 The electron density in cyanogen bromide is shifted away from the carbon atom, making it unusually
The electron density in cyanogen bromide is shifted away from the carbon atom, making it unusually electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
, and towards the more electronegative bromine and nitrogen. This leaves the carbon particularly vulnerable to attack by a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, and the cleavage reaction begins with a nucleophilic acyl substitution reaction in which bromine is ultimately replaced by the sulfur in methionine. This attack is followed by the formation of a five-membered ring as opposed to a six-membered ring, which would entail the formation of a double bond in the ring between nitrogen and carbon. This double bond would result in a rigid ring conformation, thereby destabilizing the molecule. Thus, the five-membered ring is formed so that the double bond is outside the ring, as shown in the figure.
Although the nucleophilic sulfur in methionine is responsible for attacking BrCN, the sulfur in cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, sometime ...
does not behave similarly. If the sulfur in cysteine attacked cyanogen bromide, the bromide ion would deprotonate the cyanide adduct, leaving the sulfur uncharged and the beta carbon of the cysteine not electrophilic. The strongest electrophile would then be the cyanide nitrogen, which, if attacked by water, would yield cyanic acid and the original cysteine.
Reaction conditions
Cleaving proteins with BrCN requires using a buffer such as 0.1M HCl ( hydrochloric acid) or 70% (formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
). These are the most common buffers for cleavage. An advantage to HCl is that formic acid causes the formation of formyl esters, which complicates protein characterization. However, formic is still often used because it dissolves most proteins. Also, the oxidation of methionine to methionine sulfoxide
Methionine sulfoxide is the organic compound with the formula CH3S(O)CH2CH2CH(NH2)CO2H. It is an amino acid that occurs naturally although it is formed post-translationally.
Redox, Oxidation of the sulfur of methionine results in methionine sulf ...
, which is inert to BrCN attack, occurs more readily in HCl than in formic acid, possibly because formic acid is a reducing acid. Alternative buffers for cleavage include guanidine or urea in HCl because of their ability to unfold proteins, thereby making methionine more accessible to BrCN.
Note that water is required for normal peptide bond cleavage of the iminolactone intermediate. In formic acid, cleavage of Met-Ser
Ser or SER may refer to:
Places
* Ser, a village in Bogdand Commune, Satu Mare County, Romania
* Serpens (Ser), an astronomical constellation of the northern hemisphere
* Serres, known as Ser in Serbian, a city in Macedonia, Greece
Organization ...
and Met- Thr bonds is enhanced with increased water concentration because these conditions favor the addition of water across the imine rather than reaction of the side chain hydroxyl with the imine. Lowered pH tends to increase cleavage rates by inhibiting methionine side chain oxidation.
Side reactions
When methionine is followed byserine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
or threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO� ...
, side reactions can occur that destroy the methionine without peptide bond cleavage
In chemistry, bond cleavage, or bond fission, is the splitting of chemical bonds. This can be generally referred to as dissociation when a molecule is cleaved into two or more fragments.
In general, there are two classifications for bond cleavag ...
. Normally, once the iminolactone is formed (refer to figure), water and acid can react with the imine to cleave the peptide bond, forming a homoserine lactone and new C-terminal peptide. However, if the adjacent amino acid to methionine has a hydroxyl or sulfhydryl
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
group, this group can react with the imine to form a homoserine without peptide bond cleavage. These two cases are shown in the figure.
Organic synthesis
Cyanogen bromide is a common reagent inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. As stated earlier, the reagent is prone to attack by nucleophiles such as amines and alcohols because of the electrophilic carbon. In the synthesis of cyanamides and dicyanamides, primary and secondary amines react with BrCN to yield mono- and dialkylcyanamides, which can further react with amines and hydroxylamine to yield guanidines and hydroxyguanidines. In the von Braun reaction, tertiary amines react with BrCN to yield disubstituted cyanamides and an alkyl bromide. Cyanogen bromide can be used to prepare aryl nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
s, nitriles, anhydrides, and cyanates. It can also serve as a cleaving agent.
Cyanogen bromide is used in the synthesis of 4-methylaminorex
4-Methylaminorex (4-MAR, 4-MAX) is a stimulant drug of the 2-amino-5-aryloxazoline class that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a s ...
("ice") and viroxime.
Toxicity, storage, and deactivation
Cyanogen bromide can be stored under dry conditions at 2 to 8 °C for extended periods. Cyanogen bromide is volatile, and readily absorbed through the skin orgastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organ (biology), organs of the digestive syste ...
. Therefore, toxic exposure may occur by inhalation, physical contact, or ingestion. It is acutely toxic, causing a variety of nonspecific symptoms
Signs and symptoms are the observed or detectable signs, and experienced symptoms of an illness, injury, or condition. A sign for example may be a higher or lower temperature than normal, raised or lowered blood pressure or an abnormality showin ...
. Exposure to even small amounts may cause convulsions or death. LD50 orally in rats is reported as 25–50 mg/kg.
The recommended method to deactivate cyanogen bromide is with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
and bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
. The aqueous alkali hydroxide instantly hydrolyzes (CN)Br to alkali cyanide and bromide. The cyanide can then be oxidized by sodium or calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Thi ...
to the less toxic cyanate ion. Note that deactivation is extremely exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
and may be explosive.
References
Further reading
* *External links
* * {{Cyanides Bromine compounds Cyano compounds Nonmetal halides Blood agents Lachrymatory agents Pseudohalogens