Büchner–Curtius–Schlotterbeck Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Buchner–Curtius–Schlotterbeck reaction is the  The reaction yields two possible
The reaction yields two possible
 The reaction is then completed either by the reformation of the carbonyl through an
The reaction is then completed either by the reformation of the carbonyl through an  The epoxide product is formed by an intramolecular
The epoxide product is formed by an intramolecular 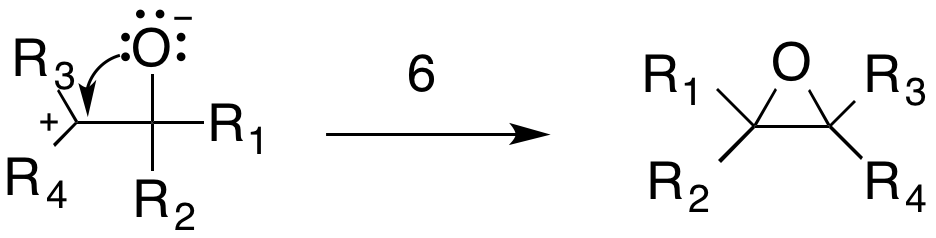 This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between
This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between
 The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.
The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.

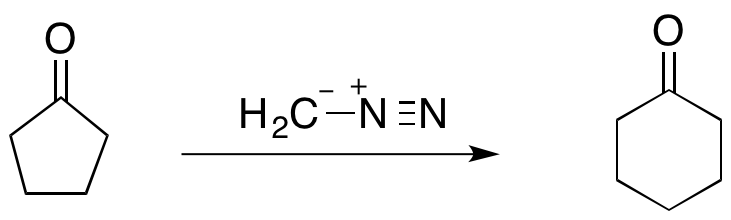 An acyl-
An acyl- Acyl-
Acyl- The Büchner–Curtius–Schlotterbeck reaction can also be used to
The Büchner–Curtius–Schlotterbeck reaction can also be used to 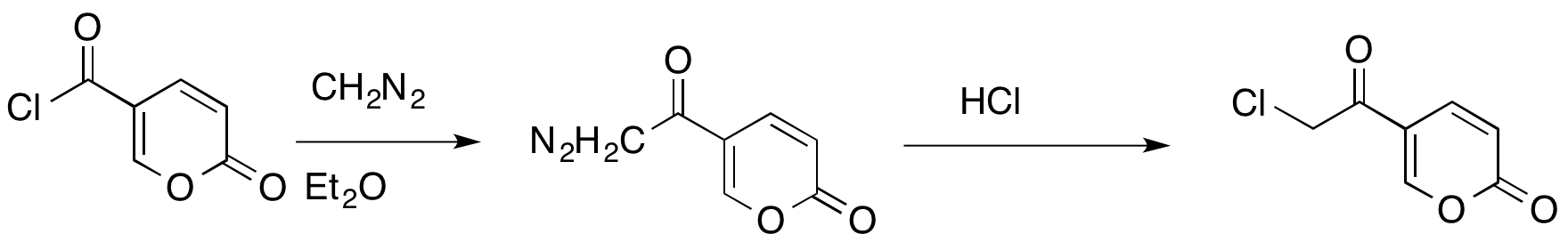 It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).
It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).

reaction
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
*Chain reaction (disambiguation).
Biology and me ...
of aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
or ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
with aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
diazo
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes ...
alkanes to form homologated ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
. It was first described by Eduard Buchner
Eduard Buchner (; 20 May 1860 – 13 August 1917) was a German chemist and zymologist, awarded the 1907 Nobel Prize in Chemistry for his work on fermentation.
Biography
Early years
Buchner was born in Munich to a physician and Doctor Extraor ...
and Theodor Curtius
''Geheimrat'' Julius Wilhelm Theodor Curtius (27 May 1857 – 8 February 1928) was professor of Chemistry at Heidelberg University and elsewhere. He published the Curtius rearrangement in 1890/1894 and also discovered diazoacetic acid, hydra ...
in 1885 and later by Fritz Schlotterbeck in 1907. Two German chemists also preceded Schlotterbeck in discovery of the reaction, Hans von Pechmann
Hans von Pechmann (1 April 1850 – 19 April 1902) was a German chemist, renowned for his discovery of diazomethane in 1894. Pechmann condensation and Pechmann pyrazole synthesis. He also first prepared 1,2-diketones (e.g., diacetyl), acetonedicarb ...
in 1895 and Viktor Meyer
Viktor Meyer (8 September 18488 August 1897) was a German chemist and significant contributor to both organic and inorganic chemistry. He is best known for inventing an apparatus for determining vapour densities, the Viktor Meyer apparatus, and ...
in 1905. The reaction has since been extended to the synthesis of β-keto esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are ...
from the condensation between aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
and diazo
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes ...
esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are ...
. The general reaction scheme is as follows:
 The reaction yields two possible
The reaction yields two possible carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
compounds (I and II) along with an epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
(III). The ratio of the products is determined by the reactant used and the reaction conditions.
Reaction Mechanism
The general mechanism is shown below. The resonating arrow (1) shows aresonance contributor
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ...
of the diazo compound with a lone pair of electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
on the carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
adjacent to the nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
. The diazo compound then does a nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
on the carbonyl-containing compound (nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
), producing a tetrahedral intermediate
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a c ...
(2). This intermediate decomposes by the evolution of nitrogen gas forming the tertiary carbocation intermediate (3).
 The reaction is then completed either by the reformation of the carbonyl through an
The reaction is then completed either by the reformation of the carbonyl through an 1,2-rearrangement A 1,2-rearrangement or 1,2-migration or 1,2-shift or Frank C. Whitmore, Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms ...
or by the formation of the epoxide. There are two possible carbonyl products: one formed by migration of R1 (4) and the other by migration of R2 (5). The relative yield of each possible carbonyl is determined by the migratory preferences of the R-groups.
 The epoxide product is formed by an intramolecular
The epoxide product is formed by an intramolecular addition reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct)..
Addition reactions are limited to chemical compounds that have multiple bonds, such as ...
in which a lone pair from the oxygen attacks the carbocation (6).
 This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between
This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost u ...
and various ketones have shown that the overall reaction follows second order kinetics. Additionally, the reactivity of two series of ketones are in the orders Cl3CCOCH3 > CH3COCH3 > C6H5COCH3 and cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexan ...
> cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclopenta ...
> cycloheptanone
Cycloheptanone, (CH2)6CO, is a cyclic ketone also referred to as suberone. It is a colourless volatile liquid. Cycloheptanone is used as a precursor for the synthesis of pharmaceuticals.
Synthesis
In 1836, French chemist Jean-Baptiste Boussinga ...
> cyclooctanone
Cyclooctanone is an organic compound with the formula . It is a waxy white solid that can be prepared by Jones oxidation of cyclooctanol. It can also be produced by ketonization reaction starting with azelaic acid
Azelaic acid (AzA) is an orga ...
. These orders of reactivity are the same as those observed for reactions that are well established as proceeding through nucleophilic attack on a carbonyl group.
Scope and variation
The reaction was originally carried out indiethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
and routinely generated high yields due to the inherent irreversibly of the reaction caused by the formation of nitrogen gas
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh ...
. Though these reactions can be carried out at room temperature, the rate does increase at higher temperatures. Typically, the reaction is carried out at less than refluxing temperatures. The optimal reaction temperature is determined by the specific diazoalkane used. Reactions involving diazomethanes with alkyl or aryl substituents are exothermic at or below room temperature. Reactions involving diazomethanes with acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC n ...
or aroyl substituents require higher temperatures. The reaction has since been modified to proceed in the presence of Lewis acids
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
and common organic solvents such as THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
and dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
. Reactions generally run at room temperature for about an hour, and the yield ranges from 70%-80% based on the choice of Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
and solvent.
Steric Effects
Steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
of the alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
substituents on the carbonyl reactant have been shown to affect both the rates
Rate or rates may refer to:
Finance
* Rates (tax), a type of taxation system in the United Kingdom used to fund local government
* Exchange rate, rate at which one currency will be exchanged for another
Mathematics and science
* Rate (mathema ...
and yields of Büchner–Curtius–Schlotterbeck reaction. Table 1 shows the percent yield of the ketone and epoxide products as well as the relative rates of reaction for the reactions between several methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
ketones and diazomethane.
The observed decrease in rate and increase in epoxide yield as the size of the alkyl group becomes larger indicates a steric effect.
Electronic Effects
Ketones and aldehydes with electron-withdrawing substituents react more readily with diazoalkanes than those bearing electron-donating substituents (Table 2). In addition to accelerating the reaction, electron-withdrawing substituents typically increase the amount of epoxide produced (Table 2). The effects of substituents on the diazoalkanes is reversed relative to the carbonyl reactants: electron-withdrawing substituents decrease the rate of reaction while electron-donating substituents accelerate it. For example, diazomethane is significantly more reactive thanethyl diazoacetate
Ethyl diazoacetate (N=N=CHC(O)OC2H5) is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetat ...
, though less reactive than its higher alkyl homologs (e.g. diazoethane). Reaction conditions may also affect the yields of carbonyl product and epoxide product. In the reactions of ''o''-nitrobenzaldehyde, ''p''-nitrobenzaldehyde, and phenylacetaldehyde
Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers. Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a phenyl substituent; the parent member of the phenylacetaldehyde class of compound ...
with diazomethane, the ratio of epoxide to carbonyl is increased by the inclusion of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
in the reaction mixture. The opposite influence has also been observed in the reaction of piperonal
Piperonal, also known as heliotropin, is an organic compound which is commonly found in fragrances and flavors.Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe a ...
with diazomethane, which exhibits increased carbonyl yield in the presence of methanol.
Migratory Preferences
The ratio of the two possible carbonyl products (I and II) obtained is determined by the relative migratory abilities of the carbonyl substituents (R1 and R2). In general, the R-group most capable of stabilizing the partial positive charge formed during the rearrangement migrates preferentially. A prominent exception to this general rule is hydride shifting. The migratory preferences of the carbonyl R-groups can be heavily influenced by solvent and diazoalkane choice. For example, methanol has been shown to promote aryl migration. As shown below, if the reaction of piperanol (IV) with diazomethane is carried out in the absence of methanol, the ketone obtained though a hydride shift is the major product (V). If methanol is the solvent, an aryl shift occurs to form the aldehyde (VI), which cannot be isolated as it continues to react to form the ketone (VII) and the epoxide (VIII) products. The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.
The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.

Examples in the Literature
The Büchner–Curtius–Schlotterbeck reaction can be used to facilitate one carbon ring expansions when the substrate ketone iscyclic
Cycle, cycles, or cyclic may refer to:
Anthropology and social sciences
* Cyclic history, a theory of history
* Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr.
* Social cycle, various cycles in soc ...
. For instance, the reaction of cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclopenta ...
with diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost u ...
forms cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexan ...
(shown below). The Büchner ring expansion reactions utilizing diazoalkanes have proven to be synthetically useful as they can not only be used to form 5- and 6-membered rings, but also more unstable 7- and 8-membered rings.
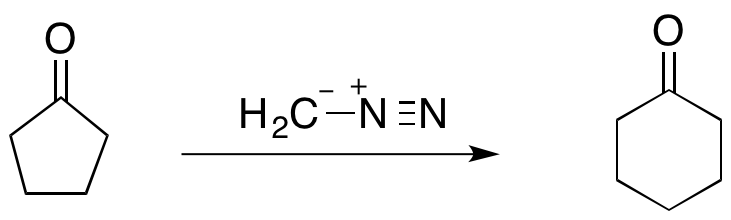 An acyl-
An acyl-diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost u ...
can react with an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
to form a β-diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls ...
in the presence of a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
( SnCl2 in the example shown below). β-Diketones are common biological products, and as such, their synthesis is relevant to biochemical research. Furthermore, the acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
ic β-hydrogens of β-diketones are useful for broader synthetic purposes, as they can be removed by common bases.
 Acyl-
Acyl-diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost u ...
can also add to esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are ...
to form β-keto esters, which are important for fatty acid synthesis
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is conve ...
. As mentioned above, the acidic β-hydrogens also have productive functionality
Function or functionality may refer to:
Computing
* Function key, a type of key on computer keyboards
* Function model, a structured representation of processes in a system
* Function object or functor or functionoid, a concept of object-oriente ...
.
 The Büchner–Curtius–Schlotterbeck reaction can also be used to
The Büchner–Curtius–Schlotterbeck reaction can also be used to insert Insert may refer to:
*Insert (advertising)
*Insert (composites)
*Insert (effects processing)
*Insert (filmmaking)
*Insert key on a computer keyboard, used to switch between insert mode and overtype mode
*Insert (molecular biology)
*Insert (SQL)
*Fi ...
a methylene bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of ...
between a carbonyl carbon and a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
of an acyl halide
In organic chemistry, an acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid by replacing a hydroxyl group () with a halide group (, where X is a halogen).
If the acid is a carboxylic acid (), the compound c ...
. This reaction allows conservation of the carbonyl and halide functionalities.
 It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).
It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).

References
{{DEFAULTSORT:Buchner-Curtius-Schlotterbeck reaction Organic reactions Name reactions