|
Cyclooctanone
Cyclooctanone is an organic compound with the formula . It is a waxy white solid that can be prepared by Jones oxidation of cyclooctanol. It can also be produced by ketonization reaction starting with azelaic acid Azelaic acid (AzA) is an organic compound with the formula HOOC(CH2)7 COOH. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a precursor to diverse industrial products including polymers an .... References {{Organic-chem-stub Cycloalkanones Eight-membered rings ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jones Oxidation
The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent. Jones reagent is a solution prepared by dissolving chromium trioxide in aqueous sulfuric acid. To effect a Jones oxidation, this acidic mixture is then added to an acetone solution of the substrate. Alternatively, potassium dichromate can be used in place of chromium trioxide. The oxidation is very rapid and quite exothermic. Yields are typically high. The reagent is convenient and cheap. However, Cr(VI) compounds are carcinogenic, which deters the use of this methodology. Stoichiometry and mechanism Jones reagent will convert primary and secondary alcohols to aldehydes and ketones, respectively. Depending on the reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
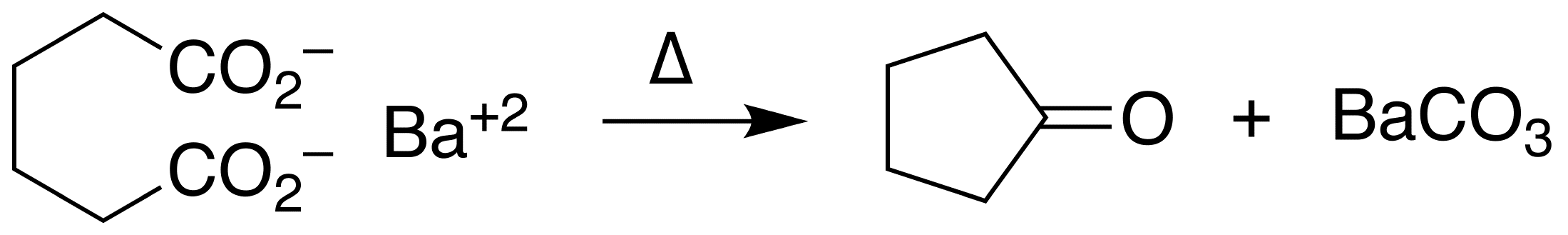
Ketonization
In organic chemistry, ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid () to a symmetric ketone () by the application of heat with expulsion of one equivalent of water () and one equivalent of carbon dioxide (): :\ce\mathbf + \ce\mathbf \longrightarrow \ce\mathbf + \ce Bases promote this reaction. The reaction mechanism likely involves nucleophilic attack of the alpha-carbon of one acid group on the other acid group's carbonyl (), possibly as a concerted reaction with the decarboxylation. The initial formation of an intermediate carbanion via decarboxylation of one of the acid groups prior to the nucleophilic attack has been proposed, but is unlikely since the byproduct resulting from the carbanion's protonation by the acid has never been reported. This reaction is different from oxidative decarboxylation, which proceeds through a radical mechanism and is charact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azelaic Acid
Azelaic acid (AzA) is an organic compound with the formula HOOC(CH2)7 COOH. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a precursor to diverse industrial products including polymers and plasticizers, as well as being a component of a number of hair and skin conditioners. AzA inhibits tyrosinase. Production Azelaic acid is industrially produced by the ozonolysis of oleic acid. The side product is nonanoic acid. It is produced naturally by ''Malassezia furfur'' (also known as ''Pityrosporum ovale''), a yeast that lives on normal skin. The bacterial degradation of nonanoic acid gives azelaic acid. Biological function In plants, azelaic acid serves as a "distress flare" involved in defense responses after infection. It serves as a signal that induces the accumulation of salicylic acid, an important component of a plant's defensive response. Applications Polymers and related materials Esters of this dicarboxylic acid f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |