Boronium on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 A borenium ion is an
A borenium ion is an
 Borenium ions can be made from tetracoordinate Lewis acid-base adducts of boron halides. In this method, halide abstraction by a
Borenium ions can be made from tetracoordinate Lewis acid-base adducts of boron halides. In this method, halide abstraction by a  Similar to the halide abstraction method, borenium ions can be made through abstraction of a hydride from a tetracoordinate boron complex.
Similar to the halide abstraction method, borenium ions can be made through abstraction of a hydride from a tetracoordinate boron complex.
 Displacement of a ligand from a neutral tricoordinate boron halide by a neutral donor such as pyridine results in the generation of a borenium cation. For this reaction to yield the desired borenium cation, the ligand must be a good leaving group and the neutral donor must have enough steric bulk that nucleophilic dissociation is favored over Lewis acid-base adduct formation with the neutral BR3 starting material, as demonstrated by competition experiments.
Displacement of a ligand from a neutral tricoordinate boron halide by a neutral donor such as pyridine results in the generation of a borenium cation. For this reaction to yield the desired borenium cation, the ligand must be a good leaving group and the neutral donor must have enough steric bulk that nucleophilic dissociation is favored over Lewis acid-base adduct formation with the neutral BR3 starting material, as demonstrated by competition experiments.
 Aminoboranes can be protonated by various acids to make borenium ions. This synthetic method was developed in 1983 by Narula and Noth who used triflic acid to protonate 1,3-dimethyl-2-(dimethylamino)-1,3,2-diazaborolidine; however, they were unable to crystallize and structurally characterize this particular cation.
Protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions that can be used as enantioselective Diels–Alder catalysts. These N-protonated borenium species have been characterized by
Aminoboranes can be protonated by various acids to make borenium ions. This synthetic method was developed in 1983 by Narula and Noth who used triflic acid to protonate 1,3-dimethyl-2-(dimethylamino)-1,3,2-diazaborolidine; however, they were unable to crystallize and structurally characterize this particular cation.
Protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions that can be used as enantioselective Diels–Alder catalysts. These N-protonated borenium species have been characterized by



 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a boranylium ion is an inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
cation with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
, where R represents a non-specific substituent. Being electron-deficient, boranylium ions form adducts with Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s. Boranylium ions have historical names that depend on the number of coordinated ligands:
*: borinium
*: borenium
*: boronium
Borenium ions
inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
cation with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. In this class of molecules, the electron-deficient boron center has two valence electrons involved in sigma bonding with two ligands, while the third ligand is a two-electron donor such that the overall charge of the complex is +1. Depending on the nature of the ligands around the central boron, this positive charge can be localized on the boron center or delocalized across the entire molecule. Borenium ions can be made in a number of different ways and are of interest for applications in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
and catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
.
Synthesis
Synthetic methods for preparing borenium ions include halide abstraction, nucleophilic dissociation, and protic addition to aminoboranes.Halide or hydride abstraction
 Borenium ions can be made from tetracoordinate Lewis acid-base adducts of boron halides. In this method, halide abstraction by a
Borenium ions can be made from tetracoordinate Lewis acid-base adducts of boron halides. In this method, halide abstraction by a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
such as AlCl3 results in a borenium cation and AlCl4− anion. The first borenium ion to be isolated and characterized was made by Ryschkewitsch and Wiggins in 1970 using this method. They found that aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
dissolved in dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
in the presence of the adduct of 4-methylpyridine
4-Methylpyridine is the organic compound with the formula CH3C5H4N. It is one of the three isomers of methylpyridine. This pungent liquid is a building block for the synthesis of other heterocyclic compounds. Its conjugate acid, the 4-methylpyrid ...
and BCl3. A positive charge on boron was then inferred from proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the struc ...
spectroscopy.
Nucleophilic dissociation
 Displacement of a ligand from a neutral tricoordinate boron halide by a neutral donor such as pyridine results in the generation of a borenium cation. For this reaction to yield the desired borenium cation, the ligand must be a good leaving group and the neutral donor must have enough steric bulk that nucleophilic dissociation is favored over Lewis acid-base adduct formation with the neutral BR3 starting material, as demonstrated by competition experiments.
Displacement of a ligand from a neutral tricoordinate boron halide by a neutral donor such as pyridine results in the generation of a borenium cation. For this reaction to yield the desired borenium cation, the ligand must be a good leaving group and the neutral donor must have enough steric bulk that nucleophilic dissociation is favored over Lewis acid-base adduct formation with the neutral BR3 starting material, as demonstrated by competition experiments.
Protic addition to aminoboranes
 Aminoboranes can be protonated by various acids to make borenium ions. This synthetic method was developed in 1983 by Narula and Noth who used triflic acid to protonate 1,3-dimethyl-2-(dimethylamino)-1,3,2-diazaborolidine; however, they were unable to crystallize and structurally characterize this particular cation.
Protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions that can be used as enantioselective Diels–Alder catalysts. These N-protonated borenium species have been characterized by
Aminoboranes can be protonated by various acids to make borenium ions. This synthetic method was developed in 1983 by Narula and Noth who used triflic acid to protonate 1,3-dimethyl-2-(dimethylamino)-1,3,2-diazaborolidine; however, they were unable to crystallize and structurally characterize this particular cation.
Protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions that can be used as enantioselective Diels–Alder catalysts. These N-protonated borenium species have been characterized by NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
.
Other methods
Borenium ions can also be made through other methods such as the addition of base to a dicoordinate borinium ion or by metathesis with salts with weakly coordinating anions such as Ag l[OC(CF3)3sub>4.html" ;"title="C(CF3)3.html" ;"title="l[OC(CF3)3">l[OC(CF3)3sub>4">C(CF3)3.html" ;"title="l[OC(CF3)3">l[OC(CF3)3sub>4or Li l[OC(CF3)3sub>4].Structure and electronics
A number of borenium ions have been structurally characterized through x-ray crystallography. The structures of borenium ions generally have two short bonds and one longer bond which is characteristic of adative bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal io ...
. The electron-deficient nature of the boron center of many borenium ions has been confirmed by computational and experimental studies. A Natural Population Analysis treatment of many borenium ions show that the boron center does indeed carry a significant positive charge. For example, the BH2NH3+ cation has a natural charge of +0.687 on boron.
Depending on the nature of the ligands around the central boron, this positive charge can be localized on the boron center or delocalized across the entire molecule. In some cases, pi-donating ligands arranged in the plane of the boron's empty p orbital can act to stabilize the electron deficiency of the boron. Density functional theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
(DFT) calculations of isolable borenium ions show that the strongly Lewis acidic boron can be stabilized by pi-donation from aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
substituents such as pyridine.

N-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
s (NHCs) can also be used to stabilize borenium ions through pi-conjugation, albeit acting as weaker pi-donors than neutral N-donors. The interaction energy between a BH2+ fragment and various NHCs has been calculated using the extended transition state method for energy decomposition analysis combined with the natural orbitals for chemical valence (NOCV) theory. This analysis showed a net pi-donating effect of the NHC ligand – in this case, the positive charge is delocalized over the entire pi system rather than localized on the boron.
In other cases the dative ligand has been observed to be twisted out of the BR3 plane due to steric crowding. This nonplanar geometry leads to a reduction in pi-donation to the boron center, making it even more electron-deficient. It has been found that increased localization of charge on the boron increases the Lewis acidity of the borocation. The Gutmann–Beckett method
In chemistry, the Gutmann–Beckett method is an experimental procedure used by chemists to assess the Lewis acidity of molecular species. Triethylphosphine oxide (, TEPO) is used as a probe molecule and systems are evaluated by 31P-NMR spectr ...
has been used by many researchers in this field to benchmark the Lewis acidities of these cations.
Early crystal structures of borenium cations indicate that the corresponding anion is non-coordinating. Further studies have shown that the reactivity of borocations is highly tied to the identity of its counter ion. In catalytic applications, weakly coordinating anions have allowed for the most active borenium catalysts. A commonly used counter ion for borenium cations is tetrakis(pentafluorophenyl)borate, B(C6F5)4−; however, other counterions such as AlCl4−, halides, and triflate are also possible. The synthetic viability of a borenium ion is often determined by its reactivity relative to its counterion. Halides are often unable to stabilize borenium ions, preferring instead to coordinate to the boron center to make a tetracoordinate species. A systematic evaluation of counterion effects on the synthetic viability of NHC-dicholoroborenium ions was conducted by Muthaiah and coworkers in 2013.
Reactivity and applications
Borenium ions are highly Lewis acidic. Their Lewis acidity is of the boron atom is determined by the electronic and steric effects of its ligands.Hydrogen activation and FLP chemistry

N-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
(NHC)-stabilized borenium ions have been demonstrated to be potent metal-free H2 activation and hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
catalysts. Unlike the neutral boranes typically used in frustrated Lewis pair
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct. Many kinds of FLPs have been devised, and many simple substrates exhibi ...
(FLP) chemistry of this type, borenium ions are inherently electrophilic and do not require electron-withdrawing ligands to perform these small-molecule activations. Because electron-withdrawing substituents can hamper hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
delivery during hydrogenation catalysis, borenium ions can be more potent catalysts than neutral boron species because they are effective hydride donors. Indeed, in 2012, Stephan and coworkers were able to develop a borenium-based FLP system capable of activating H2 stoichiometrically in the presence of phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
.
In 2015, Devillard ''et al.'' synthesized a naphthyl-bridged intramolecular borenium-containing FLP capable of activating H2 with concomitant hydrogenolysis of a mesityl ligand. A second-order perturbation theory analysis of the natural bond orbitals (NBOs) of the intermediate in this reaction involved with H2 activation showed a 281.8 kcal/mol interaction between the sigma bond of H2 and the 2p orbital of the cationic boron.
Borenium ions have also been used catalytically for various hydrogenations. Stephan and coworkers were able to use a borenium ion catalyst to activate H2 catalytically to be used for imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
hydrogenation. A similar NHC-stabilized borenium ion was used to catalyze the enantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
reduction of ketimines. In this example, enantioselectivity was afforded through the use of a chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
NHC ligand.
It has been shown that the steric and electronic properties of the NHC ligand used in these borenium catalysts is of great importance to catalytic activity: NHCs that were too bulky prevented intermolecular hydride delivery and ligands that were highly electron donating weakened the borenium cation's ability to act as a Lewis acid.
Enantioselective catalysis
Borenium ions have been used as metal-freeenantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
catalysts for a number of organic transformations. An early example of such is the Corey–Itsuno reduction
The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which an achiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine ...
. One proposed mechanism for this enantioselective reduction involves the ''in situ'' generation of a borenium-like species using BH3 as a Lewis acid.
Further work on borenium ions generated from neutral oxazaborolidines has expanded the scope of their applications. In 2002, it was reported by E. J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many a ...
and coworkers that N-protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions which can catalyze the enantioselective Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a peric ...
of 1,3-dienes with 2-methacrolein or 2-bromoacrolein. This particular borenium ion could be made ''in situ'' by protonating a neutral oxazaborolidine with trifilc acid. Corey and coworkers suggest that the stereoselectivity of this reaction is a result of aldehyde-catalyst association in the pre-transition state which governs stereoselectivity. The use of borenium ions as Diels–Alder catalysts has been further extended to the use of borenium ionic liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of ...
s as catalysts for the Diels–Alder reaction by Matuszek ''et al.'' in 2017.

Electrophilic aromatic borylation
Borenium ions have also been implicated as intermediates in electrophilic aromaticborylation Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen ...
reactions. In many examples of this reaction, a catalyst is used to activate a borane, producing a highly reactive borenium ion. The formation of this highly electrophilic species drives the formation of the Wheland intermediate, a key step in the electrophilic aromatic addition mechanism. wIn 2013, Stahl ''et al.'' used a ruthenium(II) thiolate catalyst to generate borenium ions capable of effecting direct borylation of nitrogen-containing heterocycles.
In 2017, Oestreich and coworkers developed a metal-free method for effecting this transformation. In their work, B(C6H5)3 was used to activate catecholborane, generating a borenium ion capable of borylating various electron-rich heterocycles.
Hydroboration
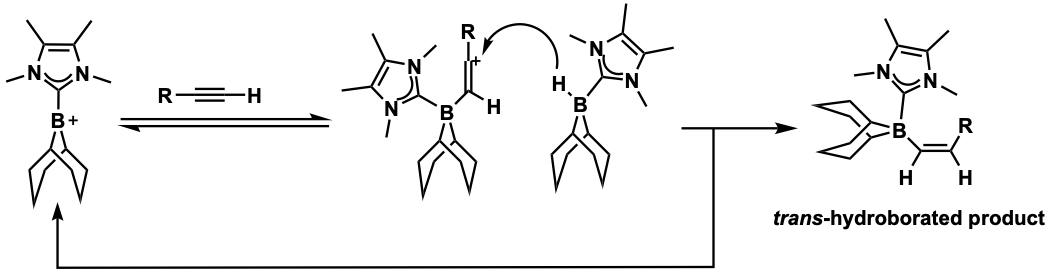
The electrophilicity of borenium ions can drive the ''trans''-hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration p ...
of alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s. In 2016, McGough ''et al.'' were able to successfully accomplish metal-free ''trans-''hydroboration with a variety of arylacetylene substrates using a borenium ion electrophile and B(C6F5)3 as a catalyst.

Polymerization catalysis
Borenium ions have been shown to formionic liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of ...
s capable of catalyzing the polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...
of polyalphaolefins (PAOs). While not yet widely adopted by industry, this technology could provide an alternative to the use of BF3, a toxic and corrosive gas, in the industrial synthesis of PAOs.
Borinium cations
Borinium ions have the formula X2sup>+, where X− is usual a bulky amide (R2N−). They have linear geometry at boron and are coordinatively unsaturated.Boronium cations
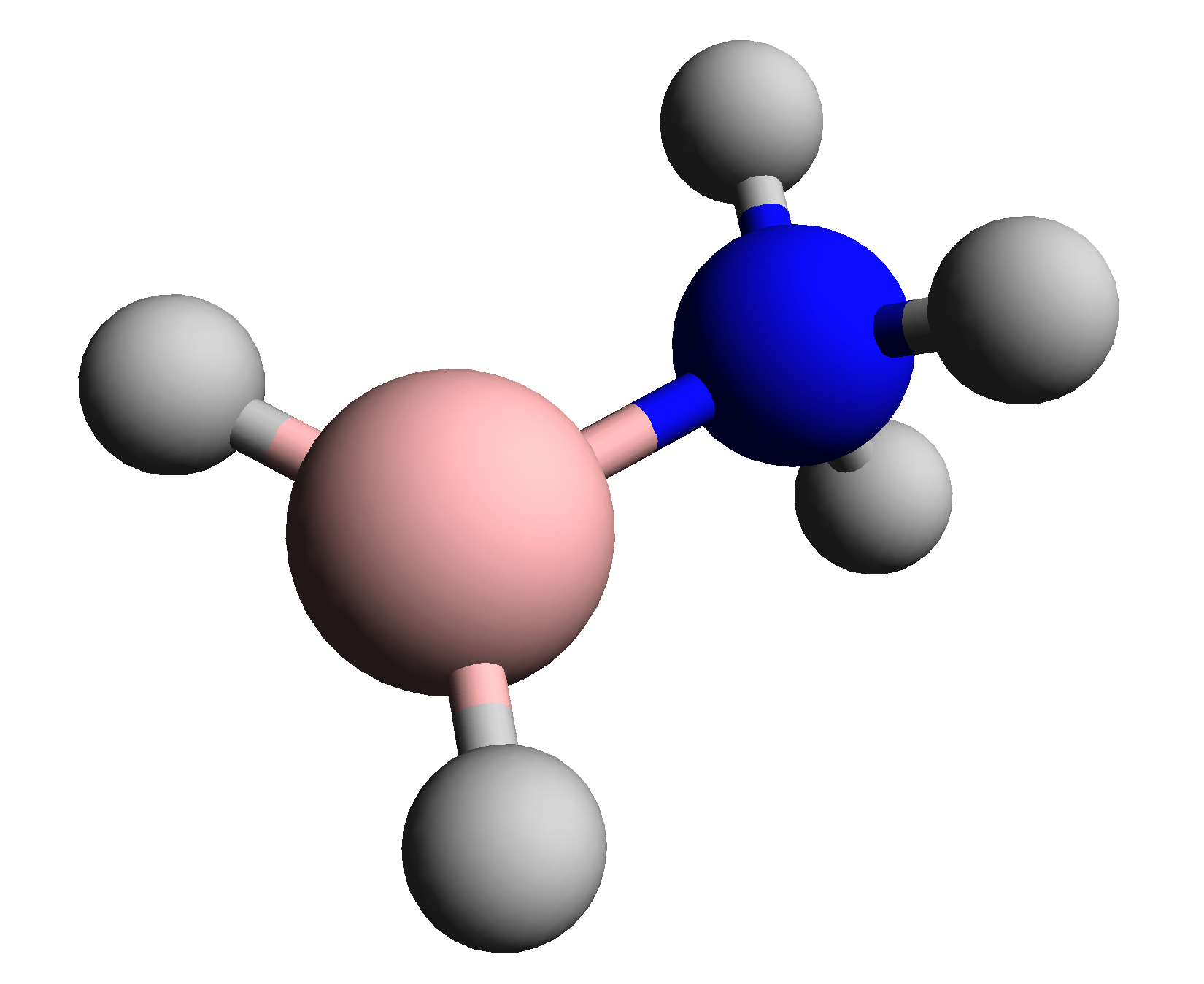
Boronium ions have the formula 2BR2sup>+ (L =Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
). Boronium ions are tetrahedral and coordinatively saturated.
A well-known example is H3N)2BH2sup>+. Reaction of diborane
Diborane(6), generally known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracte ...
with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
mainly gives 2B(NH3)2sup>+ (BH4)−.
Related boron cations
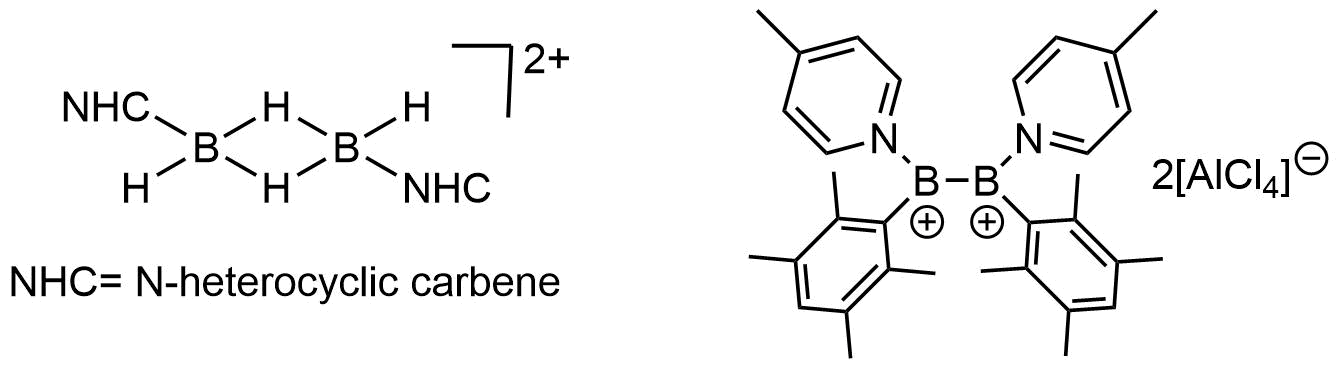
Other non-classical boron cations are mononuclear boron di- and tri-cations with formula 3BXsup>2+ and 4Bsup>3+, respectively. Other reported boron cations are dibora-dications (bis(borenium) dications), some examples are depicted below.
References
{{Reflist Boron compounds