Bismuth Phosphate on:
[Wikipedia]
[Google]
[Amazon]
Bismuth is a chemical element with the
 Beginning with Johann Heinrich Pott in 1738, Carl Wilhelm Scheele, and
Beginning with Johann Heinrich Pott in 1738, Carl Wilhelm Scheele, and
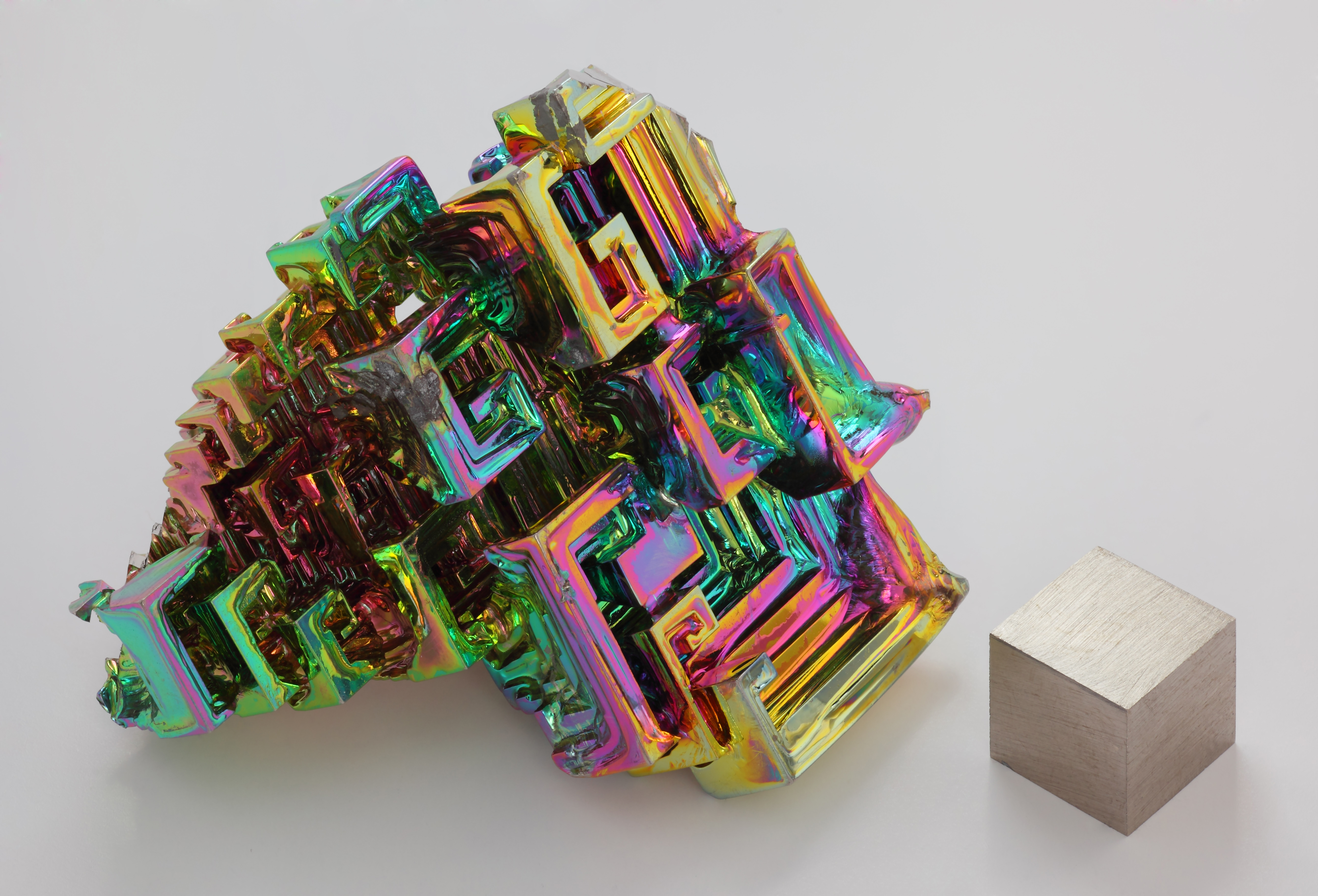
 Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of arsenic and antimony, although they are less toxic than derivatives of those lighter elements.
Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of arsenic and antimony, although they are less toxic than derivatives of those lighter elements.
 Bismuth oxychloride (BiOCl, see figure at right) and
Bismuth oxychloride (BiOCl, see figure at right) and

 In the Earth's crust, bismuth is about twice as abundant as gold. The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
The difference between mining and refining production reflects bismuth's status as a byproduct of extraction of other metals such as lead, copper, tin,
In the Earth's crust, bismuth is about twice as abundant as gold. The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
The difference between mining and refining production reflects bismuth's status as a byproduct of extraction of other metals such as lead, copper, tin,
 The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for fire sprinkler systems and fuse wire. During World War II bismuth was considered a
The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for fire sprinkler systems and fuse wire. During World War II bismuth was considered a
see "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.
 Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
 * Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth subnitrate is a component of glazes that produces an
* Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth subnitrate is a component of glazes that produces an
Lenntech.com. Retrieved on 17 December 2011. As with lead, bismuth poisoning can result in the formation of a black deposit on the gingiva, known as a bismuth line."Bismuth line"
in ''TheFreeDictionary's Medical dictionary''. Farlex, Inc. Poisoning may be treated with dimercaprol; however, evidence for benefit is unclear. Bismuth's environmental impacts are not well known; it may be less likely to bioaccumulate than some other heavy metals, and this is an area of active research.
Bismuth
at '' The Periodic Table of Videos'' (University of Nottingham)
Bismuth breaks half-life record for alpha decay
{{Authority control Chemical elements Minerals in space group 166 Native element minerals Pnictogens Post-transition metals Alchemical substances Trigonal minerals Materials that expand upon freezing Chemical elements with rhombohedral structure
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
and oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
forms are important commercial ores. The free element
In chemistry, a free element is a chemical element that is not combined with or chemical bond, chemically bonded to other elements. Examples of elements which can occur as free elements include the oxygen molecule (O) and carbon.A. Earnshaw and No ...
is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent
Iridescence (also known as goniochromism) is the phenomenon of certain surfaces that appear to gradually change color as the angle of view or the angle of illumination changes. Examples of iridescence include soap bubbles, feathers, butterfl ...
appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known.
Bismuth was long considered the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was discovered to be extremely weakly radioactive. The metal's only primordial isotope, bismuth-209, experiences alpha decay at such a minute rate that its half-life is more than a billion times the estimated age of the universe. For all but the most exotic of uses bismuth may be considered stable because of its tremendously long half-life.
Bismuth metal has been known since ancient times. Before modern analytical methods bismuth's metallurgical similarities to lead and tin often led it to be confused with those metals. The etymology of "bismuth" is uncertain. The name may come from mid-sixteenth century
The 16th century begins with the Julian year 1501 ( MDI) and ends with either the Julian or the Gregorian year 1600 ( MDC) (depending on the reckoning used; the Gregorian calendar introduced a lapse of 10 days in October 1582).
The 16th centur ...
New Latin translations of the German words or , meaning 'white mass', which were rendered as or .
Main uses
Bismuth compounds account for about half the global production of bismuth. They are used in cosmetics; pigments; and a few pharmaceuticals, notably bismuth subsalicylate, used to treat diarrhea. Bismuth's unusual propensity to expand as it solidifies is responsible for some of its uses, as in the casting of printing type. Bismuth has unusually low toxicity for a heavy metal. As the toxicity of lead and the cost of itsenvironmental remediation
Environmental remediation deals with the removal of pollution or contaminants from environmental media such as soil, groundwater, sediment, or surface water. Remedial action is generally subject to an array of regulatory requirements, and may al ...
became more apparent during the 20th century
The 20th (twentieth) century began on
January 1, 1901 ( MCMI), and ended on December 31, 2000 ( MM). The 20th century was dominated by significant events that defined the modern era: Spanish flu pandemic, World War I and World War II, nuclear ...
, suitable bismuth alloys have gained popularity as replacements for lead. Presently, around a third of global bismuth production is dedicated to needs formerly met by lead.
History and etymology
Bismuth metal has been known since ancient times and it was one of the first 10 metals to have been discovered. The name ''bismuth'' dates to around 1665 and is of uncertain etymology. The name possibly comes from obsolete German ', ', ' (early 16th century), perhaps related to Old High German ' ("white"). The New Latin ' (coined by Georgius Agricola, who Latinized many German mining and technical words) is from the German ', itself perhaps from ', meaning "white mass". The element was confused in early times with tin and lead because of its resemblance to those elements. Because bismuth has been known since ancient times, no one person is credited with its discovery. Agricola (1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties. Miners in the age of alchemy also gave bismuth the name '','' or "silver being made" in the sense of silver still in the process of being formed within the Earth. Bismuth was also known to the Incas and used (along with the usual copper and tin) in a special bronze alloy for knives.Torbern Olof Bergman
Torbern Olaf (Olof) Bergman (''KVO'') (20 March 17358 July 1784) was a Swedish chemist and mineralogist noted for his 1775 ''Dissertation on Elective Attractions'', containing the largest chemical affinity tables ever published. Bergman was the ...
, the distinctness of lead and bismuth became clear, and Claude François Geoffroy Claude François Geoffroy (1729 – 18 June 1753) was a French chemist. In 1753 he proved the chemical element bismuth to be distinct from lead, becoming the official discoverer of the element. Before this time, bismuth-containing minerals were ...
demonstrated in 1753 that this metal is distinct from lead and tin.
Characteristics

Physical characteristics
Bismuth is a brittle metal with a dark, silver-pink hue, often with aniridescent
Iridescence (also known as goniochromism) is the phenomenon of certain surfaces that appear to gradually change color as the angle of view or the angle of illumination changes. Examples of iridescence include soap bubbles, feathers, butterfl ...
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
tarnish showing many colors from yellow to blue. The spiral, stair-stepped structure of bismuth crystals is the result of a higher growth rate around the outside edges than on the inside edges. The variations in the thickness of the oxide layer that forms on the surface of the crystal cause different wavelengths of light to interfere upon reflection, thus displaying a rainbow of colors. When burned
Burned or burnt may refer to:
* Anything which has undergone combustion
* Burned (image), quality of an image transformed with loss of detail in all portions lighter than some limit, and/or those darker than some limit
* ''Burnt'' (film), a 2015 ...
in oxygen, bismuth burns with a blue flame and its oxide forms yellow fumes. Its toxicity is much lower than that of its neighbors in the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, such as lead and antimony.
No other metal is verified to be more naturally diamagnetic than bismuth. ( Superdiamagnetism is a different physical phenomenon.) Of any metal, it has one of the lowest values of thermal conductivity (after manganese, and maybe neptunium and plutonium) and the highest Hall coefficient. It has a high electrical resistivity. When deposited in sufficiently thin layers on a substrate, bismuth is a semiconductor, despite being a post-transition metal. Elemental bismuth is denser
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek language, Greek letter Rho (letter), rho), although the Latin letter ''D'' ca ...
in the liquid phase than the solid, a characteristic it shares with germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
, silicon, gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, and water. Wiberg, p. 768. Bismuth expands 3.32% on solidification; therefore, it was long a component of low-melting typesetting alloys, where it compensated for the contraction of the other alloying components to form almost isostatic bismuth-lead eutectic alloys.
Though virtually unseen in nature, high-purity bismuth can form distinctive, colorful hopper crystals. It is relatively nontoxic and has a low melting point just above 271 °C, so crystals may be grown using a household stove, although the resulting crystals will tend to be of lower quality than lab-grown crystals.
At ambient conditions, bismuth shares the same layered structure as the metallic forms of arsenic and antimony, Wiberg, p. 767. crystallizing in the rhombohedral lattice (Pearson symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example:
* Diamond structure ...
hR6, space group Rm No. 166) of the trigonal crystal system. When compressed at room temperature, this Bi-I structure changes first to the monoclinic Bi-II at 2.55 GPa, then to the tetragonal Bi-III at 2.7 GPa, and finally to the body-centered cubic Bi-V at 7.7 GPa. The corresponding transitions can be monitored via changes in electrical conductivity; they are rather reproducible and abrupt and are therefore used for calibration of high-pressure equipment.
Chemical characteristics
Bismuth is stable to both dry and moist air at ordinary temperatures. When red-hot, it reacts with water to make bismuth(III) oxide. : 2 Bi + 3 H2O → Bi2O3 + 3 H2 It reacts withfluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
to make bismuth(V) fluoride at 500 °C or bismuth(III) fluoride at lower temperatures (typically from Bi melts); with other halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s it yields only bismuth(III) halides. Wiberg, pp. 769–770.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, pp. 559–561. The trihalides are corrosive and easily react with moisture, forming oxyhalides with the formula BiOX.Suzuki
is a Japan, Japanese multinational corporation headquartered in Minami-ku, Hamamatsu, Japan. Suzuki manufactures automobiles, motorcycles, All-terrain vehicle, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs ...
, p. 9.
: 4 Bi + 6 X2 → 4 BiX3 (X = F, Cl, Br, I)
: 4 BiX3 + 2 O2 → 4 BiOX + 4 X2
Bismuth dissolves in concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
to make bismuth(III) sulfate and sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
.Suzuki
is a Japan, Japanese multinational corporation headquartered in Minami-ku, Hamamatsu, Japan. Suzuki manufactures automobiles, motorcycles, All-terrain vehicle, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs ...
, p. 8.
: 6 H2SO4 + 2 Bi → 6 H2O + Bi2(SO4)3 + 3 SO2
It reacts with nitric acid to make bismuth(III) nitrate
Bismuth(III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate. It is used in the synthesis of other bismuth compounds. It is available commercially. It is th ...
(which decomposes into nitrogen dioxide when heated).
: Bi + 6 HNO3 → 3 H2O + 3 NO2 + Bi(NO3)3
It also dissolves in hydrochloric acid, but only with oxygen present.
: 4 Bi + 3 O2 + 12 HCl → 4 BiCl3 + 6 H2O
It is used as a transmetalating agent in the synthesis of alkaline-earth metal complexes:
: 3 Ba + 2 BiPh3 → 3 BaPh2 + 2 Bi
Isotopes
The only primordial isotope of bismuth, bismuth-209, was traditionally regarded as the heaviest stable isotope, but it had long been suspected to be unstable on theoretical grounds. This was finally demonstrated in 2003, when researchers at the ''Institut d'Astrophysique Spatiale
The Institut d'astrophysique spatiale (IAS; English: Institute of Space Astrophysics) is a French research institute supporting advanced research in aerospace and astrophysics. It is located in Orsay, just south of Paris. It is a public resear ...
'' in Orsay, France, measured the alpha emission half-life of to be (3 Bq/ M g), over a billion times longer than the current estimated age of the universe. Owing to its extraordinarily long half-life, for all presently known medical and industrial applications, bismuth can be treated as if it is stable and nonradioactive. The radioactivity is of academic interest because bismuth is one of a few elements whose radioactivity was suspected and theoretically predicted before being detected in the laboratory. Bismuth has the longest known alpha decay half-life, although tellurium-128
There are 39 known isotopes and 17 nuclear isomers of tellurium (52Te), with atomic masses that range from 104 to 142. These are listed in the table below.
Naturally-occurring tellurium on Earth consists of eight isotopes. Two of these have been ...
has a double beta decay half-life of over . Bismuth's extremely long half-life means that less than approximately one-billionth of the bismuth present at the formation of the planet Earth would have decayed into thallium since then.
Several isotopes of bismuth with short half-lives occur within the radioactive disintegration chains of actinium, radium, and thorium, and more have been synthesized experimentally. Bismuth-213 is also found on the decay chain of neptunium-237 and uranium-233.
Commercially, the radioactive isotope bismuth-213 can be produced by bombarding radium with bremsstrahlung photons from a linear particle accelerator. In 1997, an antibody conjugate with bismuth-213, which has a 45-minute half-life and decays with the emission of an alpha particle, was used to treat patients with leukemia. This isotope has also been tried in cancer treatment, for example, in the targeted alpha therapy (TAT) program.
Chemical compounds
 Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of arsenic and antimony, although they are less toxic than derivatives of those lighter elements.
Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of arsenic and antimony, although they are less toxic than derivatives of those lighter elements.
Oxides and sulfides
At elevated temperatures, the vapors of the metal combine rapidly with oxygen, forming the yellow trioxide, .Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, p. 553. When molten, at temperatures above 710 °C, this oxide corrodes any metal oxide and even platinum.Krüger Krüger, Krueger or Kruger (without the umlaut Ü) are German surnames originating from '' Krüger'', meaning tavern-keeper in Low German and potter in Central German and Upper German.
The last name Krüger with umlaut dots is widespread in Germ ...
, p. 185 On reaction with a base, it forms two series of oxyanions: , which is polymeric and forms linear chains, and . The anion in is a cubic octameric anion, , whereas the anion in is tetrameric.
The dark red bismuth(V) oxide, , is unstable, liberating gas upon heating. The compound NaBiO3 is a strong oxidising agent.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, p. 578.
Bismuth sulfide, , occurs naturally in bismuth ores. It is also produced by the combination of molten bismuth and sulfur.
 Bismuth oxychloride (BiOCl, see figure at right) and
Bismuth oxychloride (BiOCl, see figure at right) and bismuth oxynitrate
Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitra ...
(BiONO3) stoichiometrically appear as simple anionic salts of the bismuthyl(III) cation (BiO+) which commonly occurs in aqueous bismuth compounds. However, in the case of BiOCl, the salt crystal forms in a structure of alternating plates of Bi, O, and Cl atoms, with each oxygen coordinating with four bismuth atoms in the adjacent plane. This mineral compound is used as a pigment and cosmetic (see below).
Bismuthine and bismuthides
Unlike the lighter pnictogens nitrogen, phosphorus, and arsenic, but similar to antimony, bismuth does not form a stable hydride. Bismuth hydride, bismuthine (), is an endothermic compound that spontaneously decomposes at room temperature. It is stable only below −60 °C. Bismuthides are intermetallic compounds between bismuth and other metals. In 2014 researchers discovered that sodium bismuthide can exist as a form of matter called a “three-dimensional topological Dirac semi-metal” (3DTDS) that possess 3DDirac fermion
In physics, a Dirac fermion is a spin-½ particle (a fermion) which is different from its antiparticle. The vast majority of fermions – perhaps all – fall under this category.
Description
In particle physics, all fermions in the standard model ...
s in bulk. It is a natural, three-dimensional counterpart to graphene with similar electron mobility
In solid-state physics, the electron mobility characterises how quickly an electron can move through a metal or semiconductor when pulled by an electric field. There is an analogous quantity for holes, called hole mobility. The term carrier mobili ...
and velocity. Graphene and topological insulators (such as those in 3DTDS) are both crystalline materials that are electrically insulating inside but conducting on the surface, allowing them to function as transistors and other electronic devices. While sodium bismuthide () is too unstable to be used in devices without packaging, it can demonstrate potential applications of 3DTDS systems, which offer distinct efficiency and fabrication advantages over planar graphene in semiconductor and spintronics applications.
Halides
Thehalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s of bismuth in low oxidation states have been shown to adopt unusual structures. What was originally thought to be bismuth(I) chloride, BiCl, turns out to be a complex compound consisting of Bi cations and BiCl and BiCl anions. The Bi cation has a distorted tricapped trigonal prismatic molecular geometry and is also found in , which is prepared by reducing a mixture of hafnium(IV) chloride and bismuth chloride
Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a covalent compound and is the common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, ...
with elemental bismuth, having the structure . Other polyatomic bismuth cations are also known, such as Bi, found in . Bismuth also forms a low-valence bromide with the same structure as "BiCl". There is a ''true'' monoiodide, BiI, which contains chains of units. BiI decomposes upon heating to the triiodide, , and elemental bismuth. A monobromide of the same structure also exists.
In oxidation state +3, bismuth forms trihalides with all of the halogens: , , , and . All of these except are hydrolyzed by water.
Bismuth(III) chloride reacts with hydrogen chloride in ether solution to produce the acid .
The oxidation state +5 is less frequently encountered. One such compound is , a powerful oxidizing and fluorinating agent. It is also a strong fluoride acceptor, reacting with xenon tetrafluoride to form the cation:
: + →
Aqueous species
In aqueous solution, the Bi ion is solvated to form the aqua ion in strongly acidic conditions. At pH > 0 polynuclear species exist, the most important of which is believed to be the octahedral complex [].Occurrence and production

 In the Earth's crust, bismuth is about twice as abundant as gold. The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
The difference between mining and refining production reflects bismuth's status as a byproduct of extraction of other metals such as lead, copper, tin,
In the Earth's crust, bismuth is about twice as abundant as gold. The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
The difference between mining and refining production reflects bismuth's status as a byproduct of extraction of other metals such as lead, copper, tin, molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
and tungsten. World bismuth production from refineries is a more complete and reliable statistic.
Bismuth travels in crude lead bullion (which can contain up to 10% bismuth) through several stages of refining, until it is removed by the Kroll-Betterton process which separates the impurities as slag, or the electrolytic Betts process The Betts electrolytic process is an industrial process for purification of lead from bullion. Lead obtained from its ores is impure because lead is a good solvent for many metals. Often these impurities are tolerated, but the Betts electrolytic p ...
. Bismuth will behave similarly with another of its major metals, copper. The raw bismuth metal from both processes contains still considerable amounts of other metals, foremost lead. By reacting the molten mixture with chlorine gas the metals are converted to their chlorides while bismuth remains unchanged. Impurities can also be removed by various other methods for example with fluxes and treatments yielding high-purity bismuth metal (over 99% Bi).
Price
 The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for fire sprinkler systems and fuse wire. During World War II bismuth was considered a
The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for fire sprinkler systems and fuse wire. During World War II bismuth was considered a strategic material
Strategic material is any sort of raw material that is important to an individual's or organization's strategic plan and supply chain management. Lack of supply of strategic materials may leave an organization or government vulnerable to disru ...
, used for solders, fusible alloys, medications and atomic research. To stabilize the market, the producers set the price at $1.25 per pound ($2.75 /kg) during the war and at $2.25 per pound ($4.96 /kg) from 1950 until 1964.
In the early 1970s, the price rose rapidly as a result of increasing demand for bismuth as a metallurgical additive to aluminium, iron and steel. This was followed by a decline owing to increased world production, stabilized consumption, and the recessions of 1980 and 1981–1982. In 1984, the price began to climb as consumption increased worldwide, especially in the United States and Japan. In the early 1990s, research began on the evaluation of bismuth as a nontoxic replacement for lead in ceramic glazes, fishing sinkers, food-processing equipment, free-machining brasses for plumbing applications, lubricating greases, and shot for waterfowl hunting.Suzuki
is a Japan, Japanese multinational corporation headquartered in Minami-ku, Hamamatsu, Japan. Suzuki manufactures automobiles, motorcycles, All-terrain vehicle, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs ...
, p. 14. Growth in these areas remained slow during the middle 1990s, in spite of the backing of lead replacement by the United States federal government, but intensified around 2005. This resulted in a rapid and continuing increase in price.Bismuth Statistics and Informationsee "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.
Recycling
Most bismuth is produced as a byproduct of other metal-extraction processes including the smelting of lead, and also of tungsten and copper. Itssustainability
Specific definitions of sustainability are difficult to agree on and have varied in the literature and over time. The concept of sustainability can be used to guide decisions at the global, national, and individual levels (e.g. sustainable livi ...
is dependent on increased recycling, which is problematic.
It was once believed that bismuth could be practically recycled from the soldered joints in electronic equipment. Recent efficiencies in solder application in electronics mean there is substantially less solder deposited, and thus less to recycle. While recovering the silver from silver-bearing solder may remain economic, recovering bismuth is substantially less so.
Next in recycling feasibility would be sizeable catalysts with a fair bismuth content, such as bismuth phosphomolybdate. Bismuth used in galvanizing, and as a free-machining metallurgical additive.
Bismuth in uses where it is dispersed most widely include certain stomach medicines ( bismuth subsalicylate), paints (bismuth vanadate
Bismuth vanadate is the inorganic compound with the formula BiVO4. It is a bright yellow solid. It is widely studied as visible light photo-catalyst with a narrow band gap of less than 2.4 eV. It is a representative of "complex inorganic colored ...
), pearlescent cosmetics ( bismuth oxychloride), and bismuth-containing bullets. Recycling bismuth from these uses is impractical.
Applications
 Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Medicines
Bismuth is an ingredient in some pharmaceuticals, although the use of some of these substances is declining. * Bismuth subsalicylate is used as an antidiarrheal; it is the active ingredient in such "pink bismuth" preparations as Pepto-Bismol, as well as the 2004 reformulation of Kaopectate. It is also used to treat some other gastro-intestinal diseases like shigellosis and cadmium poisoning. The mechanism of action of this substance is still not well documented, although an oligodynamic effect (toxic effect of small doses of heavy metal ions on microbes) may be involved in at least some cases.Salicylic acid
Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substance ...
from hydrolysis of the compound is antimicrobial for toxogenic ''E. coli,'' an important pathogen in traveler's diarrhea.
* A combination of bismuth subsalicylate and bismuth subcitrate
Bismuth subcitrate potassium is a bismuth salt used in combination with antibiotics and a proton pump inhibitor for the treatment of ''Helicobacter pylori'' infections.
A fixed-dose combination with the antibiotics metronidazole and tetracycline ...
is used to treat the bacteria causing peptic ulcers.
* Bibrocathol is an organic bismuth-containing compound used to treat eye infections.
* Bismuth subgallate, the active ingredient in Devrom, is used as an internal deodorant to treat malodor from flatulence
Flatulence, in humans, is the expulsion of gas from the intestines via the anus, commonly referred to as farting. "Flatus" is the medical word for gas generated in the stomach or bowels. A proportion of intestinal gas may be swallowed environm ...
and feces
Feces ( or faeces), known colloquially and in slang as poo and poop, are the solid or semi-solid remains of food that was not digested in the small intestine, and has been broken down by bacteria in the large intestine. Feces contain a relati ...
.
* Bismuth compounds (including sodium bismuth tartrate) were formerly used to treat syphilis. Arsenic combined with either bismuth or mercury was a mainstay of syphilis treatment from the 1920s until the advent of penicillin in 1943.
* "Milk of bismuth" (an aqueous suspension of bismuth hydroxide
Bismuth hydroxide () is non-fully characterised chemical compound of bismuth. It is produced as white flakes when alkali is added to a solution of a bismuth salt and is usually described as bismuth oxide hydrate or bismuth hydrate.
Uses
Bismuth h ...
and bismuth subcarbonate) was marketed as an alimentary cure-all in the early 20th century, and has been used to treat gastrointestinal disorders.
* Bismuth subnitrate (Bi5O(OH)9(NO3)4) and bismuth subcarbonate (Bi2O2(CO3)) are also used in medicine.
Cosmetics and pigments
Bismuth oxychloride (BiOCl) is sometimes used in cosmetics, as a pigment in paint for eye shadows, hair sprays and nail polishes. This compound is found as the mineral bismoclite and in crystal form contains layers of atoms (see figure above) that refract light chromatically, resulting in aniridescent
Iridescence (also known as goniochromism) is the phenomenon of certain surfaces that appear to gradually change color as the angle of view or the angle of illumination changes. Examples of iridescence include soap bubbles, feathers, butterfl ...
appearance similar to nacre
Nacre ( , ), also known as mother of pearl, is an organicinorganic composite material produced by some molluscs as an inner shell layer; it is also the material of which pearls are composed. It is strong, resilient, and iridescent.
Nacre is f ...
of pearl. It was used as a cosmetic in ancient Egypt and in many places since. ''Bismuth white'' (also "Spanish white") can refer to either bismuth oxychloride or bismuth oxynitrate
Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitra ...
(BiONO3), when used as a white pigment. Bismuth vanadate is used as a light-stable non-reactive paint pigment (particularly for artists' paints), often as a replacement for the more toxic cadmium sulfide yellow and orange-yellow pigments. The most common variety in artists' paints is a lemon yellow, visually indistinguishable from its cadmium-containing alternative.
Metal and alloys
Bismuth is used in metal alloys with other metals such as iron. These alloys are used in automatic sprinkler systems for fires. It forms the largest part (50%) of Rose's metal, a fusible alloy, which also contains 25–28% lead and 22–25% tin. It was also used to makebismuth bronze Bismuth bronze or bismuth brass is a copper alloy which typically contains 1-3% bismuth by weight, although some alloys contain over 6% Bi. This bronze alloy is very corrosion-resistant, a property which makes it suitable for use in environments su ...
which was used in the Bronze Age, having been found in Inca knives at Machu Picchu
Machu Picchu is a 15th-century Inca citadel located in the Eastern Cordillera of southern Peru on a mountain range.UNESCO World Heritage Centre. It is located in the Machupicchu District within Urubamba Province above the Sacred Valley, which ...
.
Lead replacement
The density difference between lead (11.32 g/cm3) and bismuth (9.78 g/cm3) is small enough that for manyballistics
Ballistics is the field of mechanics concerned with the launching, flight behaviour and impact effects of projectiles, especially ranged weapon munitions such as bullets, unguided bombs, rockets or the like; the science or art of designing and a ...
and weighting applications, bismuth can substitute for lead. For example, it can replace lead as a dense material in fishing sinkers. It has been used as a replacement for lead in shot, bullets and less-lethal riot gun ammunition. The Netherlands, Denmark, England, Wales, the United States, and many other countries now prohibit the use of lead shot for the hunting of wetland birds, as many birds are prone to lead poisoning
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. The brain is the most sensitive. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, inferti ...
owing to mistaken ingestion of lead (instead of small stones and grit) to aid digestion, or even prohibit the use of lead for all hunting, such as in the Netherlands. Bismuth-tin alloy shot is one alternative that provides similar ballistic performance to lead. (Another less expensive but also more poorly performing alternative is "steel" shot, which is actually soft iron.) Bismuth's lack of malleability
Ductility is a List of materials properties, mechanical property commonly described as a material's amenability to Drawing (manufacturing), drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a materia ...
does, however, make it unsuitable for use in expanding hunting bullets.
Bismuth, as a dense element of high atomic weight, is used in bismuth-impregnated latex shields to shield from X-ray in medical examinations, such as CTs, mostly as it is considered non-toxic.
The European Union's Restriction of Hazardous Substances Directive (RoHS) for reduction of lead has broadened bismuth's use in electronics as a component of low-melting point solders, as a replacement for traditional tin-lead solders. Its low toxicity will be especially important for solders to be used in food processing equipment and copper water pipes, although it can also be used in other applications including those in the automobile industry, in the European Union, for example.
Bismuth has been evaluated as a replacement for lead in free-machining brasses for plumbing applications, although it does not equal the performance of leaded steels.
Other metal uses and specialty alloys
Many bismuth alloys have low melting points and are found in specialty applications such as solders. Many automatic sprinklers, electric fuses, and safety devices in fire detection and suppression systems contain the eutectic In19.1-Cd5.3-Pb22.6-Sn8.3-Bi44.7 alloy that melts at This is a convenient temperature since it is unlikely to be exceeded in normal living conditions. Low-melting alloys, such as Bi-Cd-Pb-Sn alloy which melts at 70 °C, are also used in automotive and aviation industries. Before deforming a thin-walled metal part, it is filled with a melt or covered with a thin layer of the alloy to reduce the chance of breaking. Then the alloy is removed by submerging the part in boiling water.Krüger Krüger, Krueger or Kruger (without the umlaut Ü) are German surnames originating from '' Krüger'', meaning tavern-keeper in Low German and potter in Central German and Upper German.
The last name Krüger with umlaut dots is widespread in Germ ...
, p. 183.
Bismuth is used to make free-machining steel Free machining steel is steel that forms small chips when machined. This increases the machinability of the material by breaking the chips into small pieces, thus avoiding entanglement in the machinery. This enables automatic equipment to run witho ...
s and free-machining aluminium alloys for precision machining properties. It has similar effect to lead and improves the chip breaking during machining. The shrinking on solidification in lead and the expansion of bismuth compensate each other and therefore lead and bismuth are often used in similar quantities. Similarly, alloys containing comparable parts of bismuth and lead exhibit a very small change (on the order 0.01%) upon melting, solidification or aging. Such alloys are used in high-precision casting, e.g. in dentistry, to create models and molds. Bismuth is also used as an alloying agent in production of malleable irons and as a thermocouple material.
Bismuth is also used in aluminium-silicon cast alloys in order to refine silicon morphology. However, it indicated a poisoning effect on modification of strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
. Some bismuth alloys, such as Bi35-Pb37-Sn25, are combined with non-sticking materials such as mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into extremely thin elastic plates. This characteristic is described as perfect basal cleavage. Mica is ...
, glass and enamels because they easily wet them allowing to make joints to other parts. Addition of bismuth to caesium enhances the quantum yield of caesium cathodes.Krüger Krüger, Krueger or Kruger (without the umlaut Ü) are German surnames originating from '' Krüger'', meaning tavern-keeper in Low German and potter in Central German and Upper German.
The last name Krüger with umlaut dots is widespread in Germ ...
, p. 184. Sintering of bismuth and manganese powders at 300 °C produces a permanent magnet and magnetostrictive
Magnetostriction (cf. electrostriction) is a property of magnetic materials that causes them to change their shape or dimensions during the process of magnetization. The variation of materials' magnetization due to the applied magnetic field chan ...
material, which is used in ultrasonic generators and receivers working in the 10–100 kHz range and in magnetic and holographic memory devices.Suzuki
is a Japan, Japanese multinational corporation headquartered in Minami-ku, Hamamatsu, Japan. Suzuki manufactures automobiles, motorcycles, All-terrain vehicle, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs ...
, p. 15.
Other uses as compounds
 * Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth subnitrate is a component of glazes that produces an
* Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth subnitrate is a component of glazes that produces an iridescence
Iridescence (also known as goniochromism) is the phenomenon of certain surfaces that appear to gradually change color as the angle of view or the angle of illumination changes. Examples of iridescence include soap bubbles, feathers, butterfl ...
and is used as a pigment in paint.
* Bismuth telluride is a semiconductor and an excellent thermoelectric material. Bi2Te3 diodes are used in mobile refrigerators, CPU
A central processing unit (CPU), also called a central processor, main processor or just processor, is the electronic circuitry that executes instructions comprising a computer program. The CPU performs basic arithmetic, logic, controlling, and ...
coolers, and as detectors in infrared spectrophotometers.
* Bismuth oxide, in its delta form, is a solid electrolyte for oxygen. This form normally breaks down below a high-temperature threshold, but can be electrodeposited well below this temperature in a highly alkaline solution.
* Bismuth germanate
Bismuth germanium oxide or bismuth germanate is an inorganic chemical compound of bismuth, germanium and oxygen. Most commonly the term refers to the compound with chemical formula (BGO), with the cubic evlitine crystal structure, used as a sci ...
is a scintillator, widely used in X-ray and gamma ray detectors.
* Bismuth vanadate
Bismuth vanadate is the inorganic compound with the formula BiVO4. It is a bright yellow solid. It is widely studied as visible light photo-catalyst with a narrow band gap of less than 2.4 eV. It is a representative of "complex inorganic colored ...
is an opaque yellow pigment used by some artists' oil, acrylic, and watercolor paint companies, primarily as a replacement for the more toxic cadmium sulfide yellows in the greenish-yellow (lemon) to orange-toned yellow range. It performs practically identically to the cadmium pigments, such as in terms of resistance to degradation from UV exposure, opacity, tinting strength, and lack of reactivity when mixed with other pigments. The most commonly-used variety by artists' paint makers is lemon in color. In addition to being a replacement for several cadmium yellows, it also serves as a non-toxic visual replacement for the older chromate pigments made with zinc, lead, and strontium. If a green pigment and barium sulfate (for increased transparency) are added it can also serve as a replacement for barium chromate, which possesses a more greenish cast than the others. In comparison with lead chromate, it does not blacken due to hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
in the air (a process accelerated by UV exposure) and possesses a particularly brighter color than them, especially the lemon, which is the most translucent, dull, and fastest to blacken due to the higher percentage of lead sulfate required to produce that shade. It is also used, on a limited basis due to its cost, as a vehicle paint pigment.
* A catalyst for making acrylic fibers
Acrylic fibers are synthetic fibers made from a polymer (polyacrylonitrile) with an average molecular weight of ~100,000, about 1900 monomer units. For a fiber to be called "acrylic" in the US, the polymer must contain at least 85% acrylonitri ...
.
* As an electrocatalyst in the conversion of CO2 to CO.
* Ingredient in lubricating greases.
* In crackling microstars ( dragon's eggs) in pyrotechnics
Pyrotechnics is the science and craft of creating such things as fireworks, safety matches, oxygen candles, explosive bolts and other fasteners, parts of automotive airbags, as well as gas-pressure blasting in mining, quarrying, and demolition. ...
, as the oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
, subcarbonate or subnitrate.
*As catalyst for the fluorination of arylboronic pinacol esters through a Bi(III)/Bi(V) catalytic cycle, mimicking transition metals in electrophilic fluorination.
Toxicology and ecotoxicology
:''See also bismuthia, a rare dermatological condition that results from the prolonged use of bismuth.'' Scientific literature indicates that some of the compounds of bismuth are less toxic to humans via ingestion than other heavy metals (lead, arsenic, antimony, etc.) presumably due to the comparatively low solubility of bismuth salts. Its biological half-life for whole-body retention is reported to be 5 days but it can remain in the kidney for years in people treated with bismuth compounds. Bismuth poisoning can occur and has according to some reports been common in relatively recent times.Data on Bismuth's health and environmental effectsLenntech.com. Retrieved on 17 December 2011. As with lead, bismuth poisoning can result in the formation of a black deposit on the gingiva, known as a bismuth line."Bismuth line"
in ''TheFreeDictionary's Medical dictionary''. Farlex, Inc. Poisoning may be treated with dimercaprol; however, evidence for benefit is unclear. Bismuth's environmental impacts are not well known; it may be less likely to bioaccumulate than some other heavy metals, and this is an area of active research.
See also
* Lead-bismuth eutectic * List of countries by bismuth production * Bismuth minerals * Patterns in natureReferences
Bibliography
* * * *External links
* Laboratory growth of large crystals of Bismuth by Jan Kihle Crystal Pulling Laboratories, NorwayBismuth
at '' The Periodic Table of Videos'' (University of Nottingham)
Bismuth breaks half-life record for alpha decay
{{Authority control Chemical elements Minerals in space group 166 Native element minerals Pnictogens Post-transition metals Alchemical substances Trigonal minerals Materials that expand upon freezing Chemical elements with rhombohedral structure