Aromatic acids on:
[Wikipedia]
[Google]
[Amazon]
In
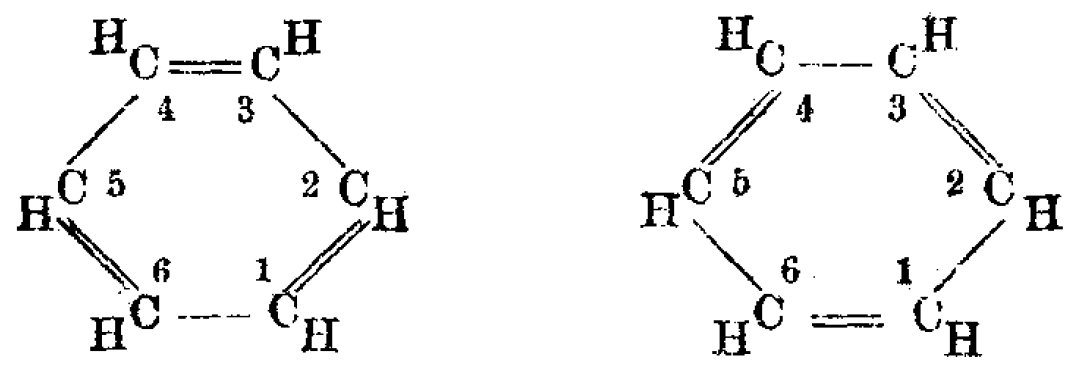
 In the 19th century, chemists found it puzzling that benzene could be so unreactive toward addition reactions, given its presumed high degree of unsaturation. The cyclohexatriene structure for benzene was first proposed by August Kekulé in 1865. Most chemists were quick to accept this structure, since it accounted for most of the known isomeric relationships of aromatic chemistry. The hexagonal structure explains why only one isomer of benzene exists and why disubstituted compounds have three isomers.
Between 1897 and 1906, J. J. Thomson, the discoverer of the electron, proposed three equivalent electrons between each pair of carbon atoms in benzene. An explanation for the exceptional stability of benzene is conventionally attributed to Sir Robert Robinson, who was apparently the first (in 1925) to coin the term ''aromatic sextet'' as a group of six electrons that resists disruption.
In fact, this concept can be traced further back, via Ernest Crocker in 1922, to Henry Edward Armstrong, who in 1890 wrote "the ixcentric affinities act within a cycle … benzene may be represented by a double ring … and when an additive compound is formed, the inner cycle of affinity suffers disruption, the contiguous carbon-atoms to which nothing has been attached of necessity acquire the ethylenic condition".
Here, Armstrong is describing at least four modern concepts. First, his "affinity" is better known nowadays as the electron, which was to be discovered only seven years later by J. J. Thomson. Second, he is describing electrophilic aromatic substitution, proceeding (third) through a
In the 19th century, chemists found it puzzling that benzene could be so unreactive toward addition reactions, given its presumed high degree of unsaturation. The cyclohexatriene structure for benzene was first proposed by August Kekulé in 1865. Most chemists were quick to accept this structure, since it accounted for most of the known isomeric relationships of aromatic chemistry. The hexagonal structure explains why only one isomer of benzene exists and why disubstituted compounds have three isomers.
Between 1897 and 1906, J. J. Thomson, the discoverer of the electron, proposed three equivalent electrons between each pair of carbon atoms in benzene. An explanation for the exceptional stability of benzene is conventionally attributed to Sir Robert Robinson, who was apparently the first (in 1925) to coin the term ''aromatic sextet'' as a group of six electrons that resists disruption.
In fact, this concept can be traced further back, via Ernest Crocker in 1922, to Henry Edward Armstrong, who in 1890 wrote "the ixcentric affinities act within a cycle … benzene may be represented by a double ring … and when an additive compound is formed, the inner cycle of affinity suffers disruption, the contiguous carbon-atoms to which nothing has been attached of necessity acquire the ethylenic condition".
Here, Armstrong is describing at least four modern concepts. First, his "affinity" is better known nowadays as the electron, which was to be discovered only seven years later by J. J. Thomson. Second, he is describing electrophilic aromatic substitution, proceeding (third) through a
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, aromaticity is a chemical property of cyclic (ring-shaped
Ring may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
:(hence) to initiate a telephone connection
Arts, entertainment and media Film and ...
), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compound
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis base. The term is used in many contexts and for many classes of chemical co ...
s having single bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of th ...
s, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compound
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
s—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning.
Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases (Purine) in RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
and DNA. An aromatic functional group or other substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
is called an aryl group.
In terms of the electronic nature of the molecule, aromaticity describes a conjugated system
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as ...
often represented in Lewis diagrams as alternating single and double bonds in a ring. In reality, the electrons represented by the double bonds in the Lewis diagram are actually distributed evenly around the ring ("delocalized"), increasing the molecule's stability. Due to the restrictions imposed by the way Lewis diagrams are drawn, the molecule cannot be represented by one diagram, but rather a hybrid of multiple different diagrams (called resonance), such as with the two resonance structures of benzene. These molecules cannot be found in either one of these representations, with the longer single bonds in one location and the shorter double bond in another (see below). Rather, the molecule exhibits all equal bond lengths in between those of single and double bonds. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.
Theory
As it is a standard for resonance diagrams, the use of a double-headed arrow indicates that two structures are not distinct entities but merely hypothetical possibilities. Neither is an accurate representation of the ''actual'' compound, which is best represented by a hybrid (average) of these structures. A C=C bond is shorter than a C−C bond. Benzene is aregular hexagon
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
—it is planar and all six carbon–carbon bonds have the same length
Length is a measure of distance. In the International System of Quantities, length is a quantity with dimension distance. In most systems of measurement a base unit for length is chosen, from which all other units are derived. In the Interna ...
, which is intermediate between that of a single
Single may refer to:
Arts, entertainment, and media
* Single (music), a song release
Songs
* "Single" (Natasha Bedingfield song), 2004
* "Single" (New Kids on the Block and Ne-Yo song), 2008
* "Single" (William Wei song), 2016
* "Single", by ...
and that of a double bond.
In a cyclic molecule with three alternating double bonds, cyclohexatriene, the bond length of the single bond would be 1.54 Å and that of the double bond would be 1.34 Å. However, in a molecule of benzene, the length of each of the bonds is 1.40 Å, indicating it to be the average of single and double bond.
A better representation is that of the circular π-bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
(Armstrong's ''inner cycle''), in which the electron density is evenly distributed through a π-bond above and below the ring. This model more correctly represents the location of electron density within the aromatic ring.
The single bonds are formed from overlap of hybridized atomic sp2-orbitals in line between the carbon nuclei—these are called σ-bonds. Double bonds consist of a σ-bond and a π-bond. The π-bonds are formed from overlap of atomic p-orbitals above and below the plane of the ring. The following diagram shows the positions of these p-orbitals:
:
Since they are out of the plane of the atoms, these orbitals can interact with each other freely, and become delocalized. This means that, instead of being tied to one atom of carbon, each electron is shared by all six in the ring. Thus, there are not enough electrons to form double bonds on all the carbon atoms, but the "extra" electrons strengthen all of the bonds on the ring equally. The resulting molecular orbital is considered to have ''π symmetry''.
:
History
The term "aromatic"
The first known use of the word "aromatic" as a ''chemical'' term—namely, to apply to compounds that contain the phenyl group—occurs in an article byAugust Wilhelm Hofmann
August Wilhelm von Hofmann (8 April 18185 May 1892) was a German chemist who made considerable contributions to organic chemistry. His research on aniline helped lay the basis of the aniline-dye industry, and his research on coal tar laid the g ...
in 1855. Hofmann used the term for a class of benzene compounds, many of which have odor
An odor (American English) or odour (English in the Commonwealth of Nations, Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is caused by one or more volatilized chemical compounds ...
s (aromas), unlike pure saturated hydrocarbons. Aromaticity as a chemical property bears no general relationship with the olfactory
The sense of smell, or olfaction, is the special sense through which smells (or odors) are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
In humans, it ...
properties of such compounds (how they smell), although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties that we recognize today are similar to unsaturated petroleum hydrocarbons like benzene. If this was indeed the earliest introduction of the term, it is curious that Hofmann says nothing about why he introduced an adjective indicating olfactory
The sense of smell, or olfaction, is the special sense through which smells (or odors) are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
In humans, it ...
character to apply to a group of chemical substances, of which only some have notable aromas. Also, some of the most odoriferous organic substances known are terpenes, which are not aromatic in the chemical sense. Terpenes and benzenoid substances do have a chemical characteristic in common, that is, higher unsaturation than many aliphatic compound
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
s, and Hofmann may not have made a distinction between the two categories. Many of the earliest-known examples of aromatic compounds, such as benzene and toluene, have distinctive pleasant smells. This property led to the term "aromatic" for this class of compounds, and hence the term "aromaticity" for the eventually discovered electronic property.
The structure of the benzene ring

Wheland intermediate
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.
For historic reasons this complex is also called a Wheland intermediate, after American chemist George ...
, in which (fourth) the conjugation
Conjugation or conjugate may refer to:
Linguistics
* Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
* Complex conjugation, the chang ...
of the ring is broken. He introduced the symbol C centered on the ring as a shorthand for the ''inner cycle'', thus anticipating Erich Clar Erich Clar (23 August 1902 in Hřensko – 27 March 1987 in Estepona) was an Austrian organic chemist who studied polycyclic aromatic hydrocarbon chemistry. He is considered as the father of that field. In 1941, he authored "Aromatische Kohlenwasser ...
's notation. It is argued that he also anticipated the nature of wave mechanics, since he recognized that his affinities had direction, not merely being point particles, and collectively having a distribution that could be altered by introducing substituents onto the benzene ring (much as the distribution of the electric charge in a body is altered by bringing it near to another body).
The quantum mechanical origins of this stability, or aromaticity, were first modelled by Hückel in 1931. He was the first to separate the bonding electrons into sigma and pi electrons.
Aromaticity of an arbitrary aromatic compound can be measured quantitatively by the nucleus-independent chemical shift (NICS) computational method
Computation is any type of arithmetic or non-arithmetic calculation that follows a well-defined model (e.g., an algorithm).
Mechanical or electronic devices (or, historically, people) that perform computations are known as '' computers''. An e ...
and aromaticity percentage methods.
Characteristics of aromatic systems
An aromatic (or aryl) ring contains a set of covalently bound atoms with specific characteristics: # A delocalized conjugated π system, most commonly an arrangement of alternating single and double bonds # Coplanar structure, with all the contributing atoms in the same plane # Contributing atoms arranged in one or more rings # A number of π delocalized electrons that is even, but not a multiple of 4. That is, 4''n'' + 2 π-electrons, where ''n'' = 0, 1, 2, 3, and so on. This is known as Hückel's rule. According to Hückel's rule, if a molecule has 4''n'' + 2 π-electrons, it is aromatic, but if it has 4''n'' π-electrons and has characteristics 1–3 above, the molecule is said to be antiaromatic. Whereas benzene is aromatic (6 electrons, from 3 double bonds), cyclobutadiene is antiaromatic, since the number of π delocalized electrons is 4, which of course is a multiple of 4. The cyclobutadienide(2−) ion, however, is aromatic (6 electrons). An atom in an aromatic system can have other electrons that are not part of the system, and are therefore ignored for the 4''n'' + 2 rule. Infuran
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
, the oxygen atom is sp2 hybridized. One lone pair is in the π system and the other in the plane of the ring (analogous to the C–H bond in the other positions). There are 6 π-electrons, so furan is aromatic.
Aromatic molecules typically display enhanced chemical stability, compared with similar non-aromatic molecules. A molecule that can be aromatic will tend to change toward aromaticity, and the added stability changes the chemistry of the molecule. Aromatic compounds undergo electrophilic aromatic substitution and nucleophilic aromatic substitution reactions, but not electrophilic addition reactions as happens with carbon–carbon double bonds.
In the presence of a magnetic field, the circulating π-electrons in an aromatic molecule produce an aromatic ring current that induces an additional magnetic field, an important effect in nuclear magnetic resonance. The NMR signal of protons in the plane of an aromatic ring are shifted substantially further down-field than those on non-aromatic sp2 carbons. This is an important way of detecting aromaticity. By the same mechanism, the signals of protons located near the ring axis are shifted upfield.
Aromatic molecules are able to interact with each other in so-called π–π stacking: The π systems form two parallel rings overlap in a "face-to-face" orientation. Aromatic molecules are also able to interact with each other in an "edge-to-face" orientation: The slight positive charge of the substituents on the ring atoms of one molecule are attracted to the slight negative charge of the aromatic system on another molecule.
Planar monocyclic molecules containing 4''n'' π-electrons are called antiaromatic and are, in general, unstable. Molecules that could be antiaromatic will tend to change from this electronic or conformation, thereby becoming non-aromatic. For example, cyclooctatetraene (COT) distorts out of planarity, breaking π overlap between adjacent double bonds. Recent studies have determined that cyclobutadiene adopts an asymmetric, rectangular configuration in which single and double bonds indeed alternate, with no resonance; the single bonds are markedly longer than the double bonds, reducing unfavorable p-orbital overlap. This reduction of symmetry lifts the degeneracy of the two formerly non-bonding molecular orbitals, which by Hund's rule forces the two unpaired electrons into a new, weakly bonding orbital (and also creates a weakly antibonding orbital). Hence, cyclobutadiene is non-aromatic; the strain of the asymmetric configuration outweighs the anti-aromatic destabilization that would afflict the symmetric, square configuration.
Hückel's rule of aromaticity treats molecules in their singlet ground states (S0). The stability trends of the compounds described here are found to be reversed in the lowest lying triplet and singlet excited states (T1 and S1), according to Baird's rule. This means that compounds like benzene, with 4''n'' + 2 π-electrons and aromatic properties in the ground state, become antiaromatic and often adopt less symmetric structures in the excited state.
Aromatic compounds
Importance
Aromatic compounds play key roles in the biochemistry of all living things. The four aromaticamino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
histidine, phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino a ...
, tryptophan, and tyrosine each serve as one of the 20 basic building-blocks of proteins. Further, all 5 nucleotides ( adenine, thymine, cytosine, guanine, and uracil) that make up the sequence of the genetic code in DNA and RNA are aromatic purines or pyrimidines. The molecule heme contains an aromatic system with 22 π-electrons. Chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
also has a similar aromatic system.
Aromatic compounds are important in industry. Key aromatic hydrocarbons of commercial interest are benzene, toluene, ''ortho''-xylene and ''para''-xylene. About 35 million tonnes are produced worldwide every year. They are extracted from complex mixtures obtained by the refining of oil or by distillation of coal tar, and are used to produce a range of important chemicals and polymers, including styrene, phenol, aniline, polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include natural ...
and nylon.
Neutral homocyclics
Benzene, as well as most other annulenes (with the exception ofcyclodecapentaene
Cyclodecapentaene or 0nnulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because va ...
, because it is non-planar) with the formula C4''n''+2H4''n''+2 where ''n'' is a natural number, such as cyclotetradecaheptaene
Cyclotetradecaheptaene, often referred to as 4nnulene, is a hydrocarbon with molecular formula C14H14, which played an important role in the development of criteria (Hückel's rule) for aromaticity, a stabilizing property of central importance in ...
(''n''=3).
Heterocyclics
In heterocyclic aromatics (heteroaromatics), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case offuran
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
) increase its reactivity. Other examples include pyridine, pyrazine, pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
, imidazole, pyrazole, oxazole, thiophene, and their benzannulated
In organic chemistry annulation (from the Latin ''anellus'' for "little ring"; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule.
:
Examples are the Robinson annulation, Danheiser annulation and cert ...
analogs ( benzimidazole, for example). In all these examples, the number of π-electrons is 6, due to the π-electrons from the double bonds as well as the two electrons from any lone pair that is in the p-orbital that is in the plane of the aromatic π system. For example, in pyridine, the five sp2-hybridized carbons each have a p-orbital that is perpendicular to the plane of the ring, and each of these p-orbitals contains one π-electron. Additionally, the nitrogen atom is also sp2-hybridized and has one electron in a p-orbital, which adds up to 6 p-electrons, thus making pyridine aromatic. The lone pair on the nitrogen is not part of the aromatic π system. Pyrrole and imidazole are both five membered aromatic rings that contain heteroatoms. In pyrrole, each of the four sp2-hybridized carbons contributes one π-electron, and the nitrogen atom is also sp2-hybridized and contributes two π-electrons from its lone pair, which occupies a p-orbital. In imidazole, both nitrogens are sp2-hybridized; the one in the double bond contributes one electron and the one which is not in the double bond and is in a lone pair contributes two electrons to the π system.
Fused aromatics and polycyclics
Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms. Examples arenaphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
, anthracene, and phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics and pesticides, e ...
. In fused aromatics, not all carbon–carbon bonds are necessarily equivalent, as the electrons are not delocalized over the entire molecule. The aromaticity of these molecules can be explained using their orbital picture. Like benzene and other monocyclic aromatic molecules, polycyclics have a cyclic conjugated pi system with p-orbital overlap above and below the plane of the ring.
Substituted aromatics
Many chemical compounds are aromatic rings with other functional groups attached. Examples include trinitrotoluene (TNT), acetylsalicylic acid (aspirin),paracetamol
Paracetamol, also known as acetaminophen, is a medication used to treat fever and mild to moderate pain. Common brand names include Tylenol and Panadol.
At a standard dose, paracetamol only slightly decreases body temperature; it is inferior ...
, and the nucleotides of DNA.
Aromatic ions
Aromatic molecules need not be neutral molecules. Ions that satisfy Huckel's rule of 4''n'' + 2 π-electrons in a planar, cyclic, conjugated molecule are considered to be aromatic ions. For example, the cyclopentadienyl anion and the cycloheptatrienylium cation are both considered to be aromatic ions, and the azulene molecule can be approximated as a combination of both. In order to convert the atom from sp3 to sp2, acarbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
, carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
, or carbon radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
must be formed. These leave sp2-hybridized carbons that can partake in the π system of an aromatic molecule. Like neutral aromatic compounds, these compounds are stable and form easily. The cyclopentadienyl anion is formed very easily and thus 1,3-cyclopentadiene is a very acidic hydrocarbon with a p''K''a of 16. Other examples of aromatic ions include the cyclopropenium
The cyclopropenium ion is the cation with the formula . It has attracted attention as the smallest example of an aromatic cation. Its salts have been isolated, and many derivatives have been characterized by X-ray crystallography. The cation and so ...
cation (2 π-electrons) and cyclooctatetraenyl dianion (10 π electrons).
Atypical aromatic compounds
Aromaticity also occurs in compounds that are not carbocyclic or heterocyclic; inorganic six-membered-ring compounds analogous to benzene have been synthesized. For example,borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this ...
is a six-membered ring composed of alternating boron and nitrogen atoms, each with one hydrogen attached. It has a delocalized π system and undergoes electrophilic substitution reactions appropriate to aromatic rings rather than reactions expected of non-aromatic molecules.
Quite recently, the aromaticity of planar rings occurring in the Zintl phase Li12Si7 was experimentally evinced by Li solid-state NMR. Metal aromaticity
Metal aromaticity or metalloaromaticity is the concept of aromaticity, found in many organic compounds, extended to metals and metal-containing compounds. The first experimental evidence for the existence of aromaticity in metals was found in alum ...
is believed to exist in certain clusters of aluminium and gallium, specifically Ga32- and Al42-, for example.
Homoaromaticity is the state of systems where conjugation is interrupted by a single sp hybridized carbon atom.
Y-aromaticity is used to describe a Y-shaped, planar (flat) molecule with resonance bonds. The concept was developed to explain the extraordinary stability and high basicity of the guanidinium cation. Guanidinium is not a ring molecule, and is cross-conjugated rather than a π system of consecutively attached atoms, but is reported to have its six π-electrons delocalized over the whole molecule. The concept is controversial and some authors emphasize different effects. This has also been suggested as the reason that the trimethylenemethane
Trimethylenemethane (often abbreviated TMM) is a chemical compound with formula . It is a neutral free molecule with two unsatisfied valence bonds, and is therefore a highly reactive free radical. Formally, it can be viewed as an isobutylene mo ...
dication is more stable than the butadienyl dication.
σ-aromaticity refers to stabilization arising from the delocalization of sigma bonds. It is often invoked in cluster chemistry and is closely related to Wade's Rule
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by ...
. Furthermore, in 2021 a σ-aromatic Th3 complex was reported, indicating that the concept of σ-aromaticity remains relevant for orbitals with principle quantum number 6.
Other symmetries
Möbius aromaticity
In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of molecular orbital theory these compounds have in common a monocyclic array of molecular orbitals in which th ...
occurs when a cyclic system of molecular orbitals, formed from pπ atomic orbitals and populated in a closed shell by 4''n'' (''n'' is an integer) electrons, is given a single half-twist to form a Möbius strip
In mathematics, a Möbius strip, Möbius band, or Möbius loop is a surface that can be formed by attaching the ends of a strip of paper together with a half-twist. As a mathematical object, it was discovered by Johann Benedict Listing and Augu ...
. A π system with 4''n'' electrons in a flat (non-twisted) ring would be antiaromatic, and therefore highly unstable, due to the symmetry of the combinations of p atomic orbitals. By twisting the ring, the symmetry of the system changes and becomes allowed (see also Möbius–Hückel concept In chemistry, the Möbius–Hückel treatment is a methodology used to predict whether a reaction is allowed or forbidden. It is often used alone with the Woodward–Hoffmann approach. The description in this article uses the plus-minus sign notat ...
for details). Because the twist can be left-handed or right-handed, the resulting Möbius aromatics are ''dissymmetric'' or chiral. But as of 2012, no Möbius aromatic molecules had been synthesized. Aromatics with two half-twists corresponding to the paradromic topologies were first suggested by Johann Listing
Johann Benedict Listing (25 July 1808 – 24 December 1882) was a German mathematician.
J. B. Listing was born in Frankfurt and died in Göttingen. He first introduced the term "topology" to replace the older term "geometria situs" (also called ...
. In one form of carbo-benzene
In organic chemistry, a ''carbo''-mer (often carbo-mer or carbomer) is an expanded molecule obtained by insertion of C2 units into a given molecule. ''Carbo''-mers differ from their templates in size but not in symmetry when each C–C single bon ...
, the ring is expanded and contains alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
and allene groups.
Spherical aromaticity In organic chemistry, spherical aromaticity is formally used to describe an unusually stable nature of some spherical compounds such as fullerenes, polyhedral boranes.
In 2000, Andreas Hirsch and coworkers in Erlangen, Germany, formulated a rule to ...
is aromaticity that occurs in fullerenes. In 2000, Andreas Hirsch and coworkers in Erlangen, Germany, formulated a rule to determine when a fullerene would be aromatic. They found that if there were 2(''n'' + 1)2 π- electrons, then the fullerene would display aromatic properties. This follows from the fact that an aromatic fullerene must have full icosahedral (or other appropriate) symmetry, so the molecular orbitals must be entirely filled. This is possible only if there are exactly 2(''n'' + 1)2 electrons, where ''n'' is a nonnegative integer.
See also
* Aromatization * Aromatic amine *List of benzo compounds
In organic chemistry the addition of the prefix benzo to the name of a chemical compound indicates the addition of an even number of carbon atoms to an unsaturated or already aromatic compound
Aromatic compounds, also known as "mono- and poly ...
* Pi interaction In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system c ...
* Avoided crossing
References
External links
* {{Authority control Physical organic chemistry