Alanine And Aspartate Metabolism on:
[Wikipedia]
[Google]
[Amazon]
Alanine (symbol Ala or A), or α-alanine, is an α-
 :
:
 Alanine is useful in loss of function experiments with respect to phosphorylation. Some techniques involve creating a library of genes, each of which has a point mutation at a different position in the area of interest, sometimes even every position in the whole gene: this is called "scanning mutagenesis". The simplest method, and the first to have been used, is so-called
Alanine is useful in loss of function experiments with respect to phosphorylation. Some techniques involve creating a library of genes, each of which has a point mutation at a different position in the area of interest, sometimes even every position in the whole gene: this is called "scanning mutagenesis". The simplest method, and the first to have been used, is so-called
amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
that is used in the biosynthesis of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s. It contains an amine group
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
, aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
α-amino acid. Under biological conditions, it exists in its zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
ic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded
In communications and information processing, code is a system of rules to convert information—such as a letter, word, sound, image, or gesture—into another form, sometimes shortened or secret, for communication through a communication ...
by all codons starting with GC (GCU, GCC, GCA, and GCG).
The L- isomer of alanine (left-handed
In human biology, handedness is an individual's preferential use of one hand, known as the dominant hand, due to it being stronger, faster or more dextrous. The other hand, comparatively often the weaker, less dextrous or simply less subject ...
) is the one that is incorporated into proteins. L-alanine is second only to leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- ...
in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in polypeptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
in some bacterial cell walls and in some peptide antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention o ...
, and occurs in the tissues of many crustacean
Crustaceans (Crustacea, ) form a large, diverse arthropod taxon which includes such animals as decapods, seed shrimp, branchiopods, fish lice, krill, remipedes, isopods, barnacles, copepods, amphipods and mantis shrimp. The crustacean group can ...
s and molluscs
Mollusca is the second-largest phylum of invertebrate animals after the Arthropoda, the members of which are known as molluscs or mollusks (). Around 85,000 extant species of molluscs are recognized. The number of fossil species is estim ...
as an osmolyte
Osmolytes are low-molecular weight organic compounds that influence the properties of biological fluids. Their primary role is to maintain the integrity of cells by affecting the viscosity, melting point, and ionic strength of the aqueous solution. ...
.
History and etymology
Alanine was first synthesized in 1850 whenAdolph Strecker
Adolph Strecker (October 21, 1822 – November 7, 1871) was a German chemist who is remembered primarily for his work with amino acids.
Life and work
Strecker was born in Darmstadt, the son of Friedrich Ludwig Strecker, an archivist working for ...
combined acetaldehyde and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
with hydrogen cyanide. The amino acid was named '' Alanin'' in German, in reference to aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, with the infix ''-an-'' for ease of pronunciation, the German ending '' -in'' used in chemical compounds being analogous to English ''-ine
''-ine'' is a suffix used in chemistry to denote two kinds of substance. The first is a chemically basic and alkaloidal substance. It was proposed by Joseph Louis Gay-Lussac in an editorial accompanying a paper by Friedrich Sertürner describing ...
''.
Structure
Alanine is analiphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
amino acid, because the side-chain connected to the α-carbon atom is a methyl group (-CH3); alanine is the simplest α-amino acid after glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
. The methyl side-chain of alanine is non-reactive and is therefore hardly ever directly involved in protein function. Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained through the diet. Alanine is found in a wide variety of foods, but is particularly concentrated in meats.
Sources
Biosynthesis
Alanine can be synthesized from pyruvate and branched chain amino acids such asvaline
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotona ...
, leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- ...
, and isoleucine.
Alanine is produced by reductive amination
Reductive amination (also known as reductive alkylation) is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered ...
of pyruvate, a two-step process. In the first step, α-ketoglutarate, ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
and NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an aden ...
are converted by glutamate dehydrogenase
Glutamate dehydrogenase (GLDH, GDH) is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typical ...
to glutamate, NAD+ and water. In the second step, the amino group of the newly-formed glutamate is transferred to pyruvate by an aminotransferase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
Function and mechanism
An amino acid co ...
enzyme, regenerating the α-ketoglutarate, and converting the pyruvate to alanine. The net result is that pyruvate and ammonia are converted to alanine, consuming one reducing equivalent
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
. Because transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential ...
reactions are readily reversible and pyruvate is present in all cells, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis, gluconeogenesis, and the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
.
Chemical synthesis
L-Alanine is produced industrially by decarboxylation of L-aspartate by the action of aspartate 4-decarboxylase. Fermentation routes to L-alanine are complicated byalanine racemase
In enzymology, an alanine racemase () is an enzyme that catalyzes the chemical reaction
:L-alanine \rightleftharpoons D-alanine
Hence, this enzyme has one substrate, L-alanine, and one product, D-alanine.
This enzyme belongs to the family o ...
.
Racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
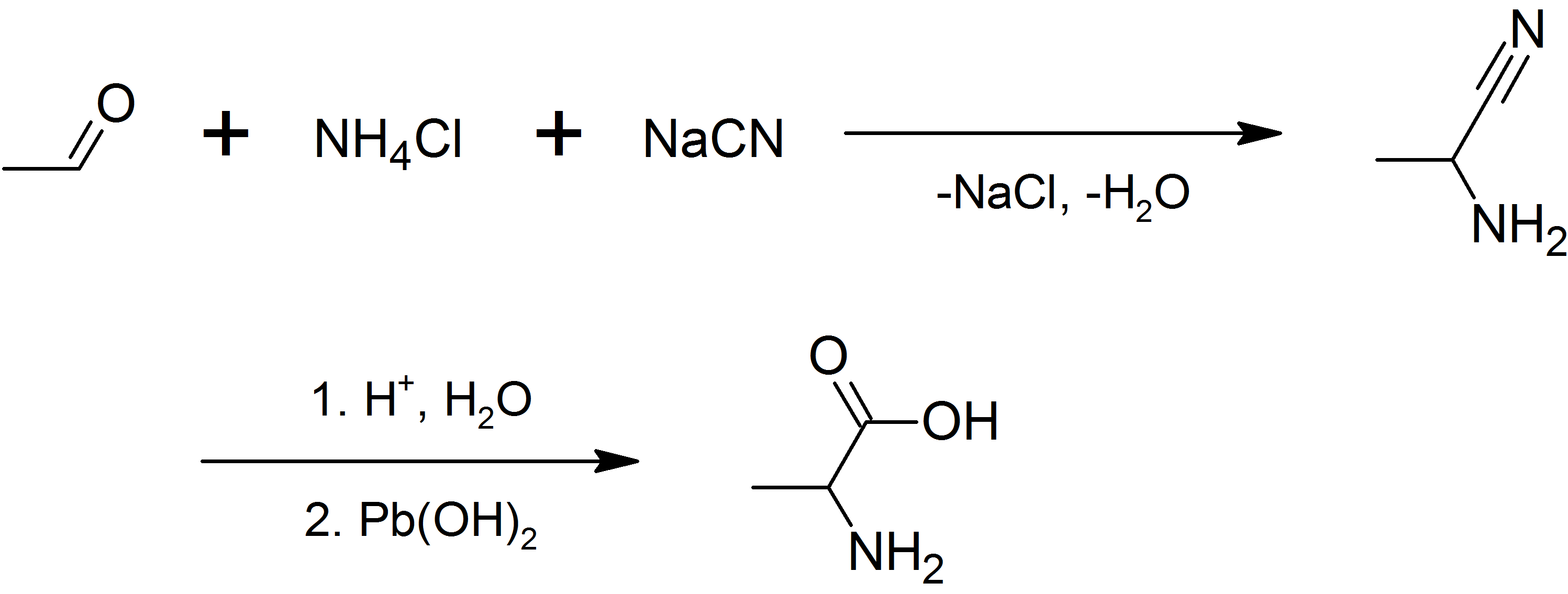
alanine can be prepared by the condensation of acetaldehyde with ammonium chloride in the presence of sodium cyanide
Sodium cyanide is a poisonous compound with the formula Na C N. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its hi ...
by the Strecker reaction
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonia in the presence of potassium cyanide. The condensation reaction yields an α- ...
, or by the ammonolysis
In chemistry, ammonolysis (/am·mo·nol·y·sis/) is the process of splitting ammonia into NH2- + H+. Ammonolysis reactions can be conducted with organic compounds to produce amines (molecules containing a nitrogen atom with a lone pair, :N), o ...
of 2-bromopropanoic acid.
: :
:
Degradation
Alanine is broken down by oxidative deamination, the inverse reaction of the reductive amination reaction described above, catalyzed by the same enzymes. The direction of the process is largely controlled by the relative concentration of the substrates and products of the reactions involved.Alanine World Hypothesis
Alanine is one of the twenty canonical α-amino acids used as building blocks (monomers) for the ribosome-mediated biosynthesis of proteins. Alanine is believed to be one of the earliest amino acids to be included in the genetic code standard repertoire. On the basis of this fact the "Alanine World" hypothesis was proposed. This hypothesis explains the evolutionary choice of amino acids in the repertoire of the genetic code from a chemical point of view. In this model the selection of monomers (i.e. amino acids) for ribosomal protein synthesis is rather limited to those Alanine derivatives that are suitable for buildingα-helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ...
or β-sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
secondary structural elements. Dominant secondary structures in life as we know it are α-helices and β-sheets and most canonical amino acids can be regarded as chemical derivatives of Alanine. Therefore, most canonical amino acids in proteins can be exchanged with Ala by point mutations while the secondary structure remains intact. The fact that Ala mimics the secondary structure preferences of the majority of the encoded amino acids is practically exploited in alanine scanning
In molecular biology, alanine scanning is a site-directed mutagenesis technique used to determine the contribution of a specific residue to the stability or function of a given protein. Alanine is used because of its non-bulky, chemically inert, ...
mutagenesis. In addition, classical X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
often employs the polyalanine-backbone model to determine three-dimensional structures of proteins using molecular replacement
Molecular replacement (or MR) is a method of solving the phase problem in X-ray crystallography. MR relies upon the existence of a previously solved protein structure which is similar to our unknown structure from which the diffraction data is de ...
- a model-based phasing
A phaser is an electronic sound processor used to filter a signal, and it has a series of troughs in its frequency-attenutation graph. The position (in Hz) of the peaks and troughs are typically modulated by an internal low-frequency oscil ...
method.
Physiological function
Glucose–alanine cycle
In mammals, alanine plays a key role inglucose–alanine cycle
The Cahill cycle, also known as the alanine cycle or glucose-alanine cycle, is the series of reactions in which amino groups and carbons from muscle are transported to the liver. It is quite similar to the Cori cycle in the cycling of nutrients b ...
between tissues and liver. In muscle and other tissues that degrade amino acids for fuel, amino groups are collected in the form of glutamate by transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential ...
. Glutamate can then transfer its amino group to pyruvate, a product of muscle glycolysis, through the action of alanine aminotransferase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first character ...
, forming alanine and α-ketoglutarate. The alanine enters the bloodstream, and is transported to the liver. The alanine aminotransferase reaction takes place in reverse in the liver, where the regenerated pyruvate is used in gluconeogenesis, forming glucose which returns to the muscles through the circulation system. Glutamate in the liver enters mitochondria and is broken down by glutamate dehydrogenase
Glutamate dehydrogenase (GLDH, GDH) is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typical ...
into α-ketoglutarate and ammonium, which in turn participates in the urea cycle
The urea cycle (also known as the ornithine cycle) is a cycle of Biochemistry, biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.
The urea cycle ...
to form urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
which is excreted through the kidneys..
The glucose–alanine cycle enables pyruvate and glutamate to be removed from muscle and safely transported to the liver. Once there, pyruvate is used to regenerate glucose, after which the glucose returns to muscle to be metabolized for energy: this moves the energetic burden of gluconeogenesis to the liver instead of the muscle, and all available ATP in the muscle can be devoted to muscle contraction. It is a catabolic pathway, and relies upon protein breakdown in the muscle tissue. Whether and to what extent it occurs in non-mammals is unclear.
Link to diabetes
Alterations in the alanine cycle that increase the levels of serumalanine aminotransferase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first character ...
(ALT) are linked to the development of type II diabetes.
Chemical properties
 Alanine is useful in loss of function experiments with respect to phosphorylation. Some techniques involve creating a library of genes, each of which has a point mutation at a different position in the area of interest, sometimes even every position in the whole gene: this is called "scanning mutagenesis". The simplest method, and the first to have been used, is so-called
Alanine is useful in loss of function experiments with respect to phosphorylation. Some techniques involve creating a library of genes, each of which has a point mutation at a different position in the area of interest, sometimes even every position in the whole gene: this is called "scanning mutagenesis". The simplest method, and the first to have been used, is so-called alanine scanning
In molecular biology, alanine scanning is a site-directed mutagenesis technique used to determine the contribution of a specific residue to the stability or function of a given protein. Alanine is used because of its non-bulky, chemically inert, ...
, where every position in turn is mutated to alanine.
Hydrogenation of alanine gives the amino alcohol
In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.
Meth ...
alaninol
Alaninol is the organic compound with the formula CH3CH(NH2)CH2OH. A colorless solid, the compound is classified as an amino alcohol. It can be generated by converting the carboxylic group of alanine to an alcohol with a strong reducing agent suc ...
, which is a useful chiral building block.
Free radical
Thedeamination
Deamination is the removal of an amino group from a molecule. Enzymes that catalyse this reaction are called deaminases.
In the human body, deamination takes place primarily in the liver, however it can also occur in the kidney. In situations of ...
of an alanine molecule produces the free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
CH3C•HCO2−. Deamination can be induced in solid or aqueous alanine by radiation that causes homolytic cleavage of the carbon–nitrogen bond..
This property of alanine is used in dosimetric measurements in radiotherapy. When normal alanine is irradiated, the radiation causes certain alanine molecules to become free radicals, and, as these radicals are stable, the free radical content can later be measured by electron paramagnetic resonance in order to find out how much radiation the alanine was exposed to. This is considered to be a biologically relevant measure of the amount of radiation damage that living tissue would suffer under the same radiation exposure. Radiotherapy treatment plans can be delivered in test mode to alanine pellets, which can then be measured to check that the intended pattern of radiation dose is correctly delivered by the treatment system.
References
{{Authority control Proteinogenic amino acids Glucogenic amino acids Glycine receptor agonists NMDA receptor agonists