AIDS Vaccine on:
[Wikipedia]
[Google]
[Amazon]
 An HIV vaccine is a potential vaccine that could be either a preventive
An HIV vaccine is a potential vaccine that could be either a preventive
 The
The
 The typical
The typical
Vaccine Research Center (VRC)
Information concerning Preventive HIV vaccine research studies
NIAID HIV vaccine site
(
Global Alliance for Vaccines and Immunization (GAVI)
International AIDS Vaccine Initiative (IAVI)
AIDS Vaccine Advocacy Coalition (AVAC)
U.S. Military HIV Research Program (MHRP)
HIV.gov - The U.S. Federal Domestic HIV/AIDS Resource
HIVtest.org - Find an HIV testing site near you
- The New York Times, March 8, 2019
Treatment Action Group
{{Authority control Prevention of HIV/AIDS Hypothetical technology
 An HIV vaccine is a potential vaccine that could be either a preventive
An HIV vaccine is a potential vaccine that could be either a preventive vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifie ...
or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV
The human immunodeficiency viruses (HIV) are two species of ''Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune ...
or treat HIV-infected individuals.
It is thought that an HIV vaccine could either induce an immune response against HIV (active vaccination approach) or consist of preformed antibodies against HIV (passive vaccination approach).
Two active vaccine regimens, studied in the RV 144 RV 144, or the Thai trial, was an HIV vaccine clinical trial that was conducted in Thailand between 2003 and 2006. It used a combination of two HIV vaccines that had each failed in earlier trials. Participants were vaccinated over the course of 24 w ...
and Imbokodo trials, showed they can prevent HIV in some individuals.
However, the protection was in relatively few individuals, and was not long lasting. For these reasons, no HIV vaccines have been licensed for the market yet.
Difficulties in development
In 1984, after it was confirmed that HIV caused AIDS, the United States Health and Human Services SecretaryMargaret Heckler
Margaret Mary Heckler (née O'Shaughnessy; June 21, 1931 – August 6, 2018) was an American politician and diplomat who represented in the United States House of Representatives from 1967 until 1983. A member of the Republican Party, she al ...
declared that a vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifie ...
would be available within two years. However, priming the adaptive immune system to recognize the viral envelope
A viral envelope is the outermost layer of many types of viruses. It protects the genetic material in their life cycle when traveling between host cells. Not all viruses have envelopes.
Numerous human pathogenic viruses in circulation are encase ...
proteins did not prevent HIV acquisition.
Many factors make the development of an HIV vaccine different from other classic vaccines (as of 1996):
* Classic vaccines mimic natural immunity against reinfection as seen in individuals recovered from infection; there are few recovered AIDS patients.
* Most vaccines protect against disease, not against infection; HIV infection may remain latent for long periods before causing AIDS.
* Most effective vaccines are whole-killed or live-attenuated organisms; killed HIV-1 does not retain antigenicity and the use of a live retrovirus vaccine raises safety issues.
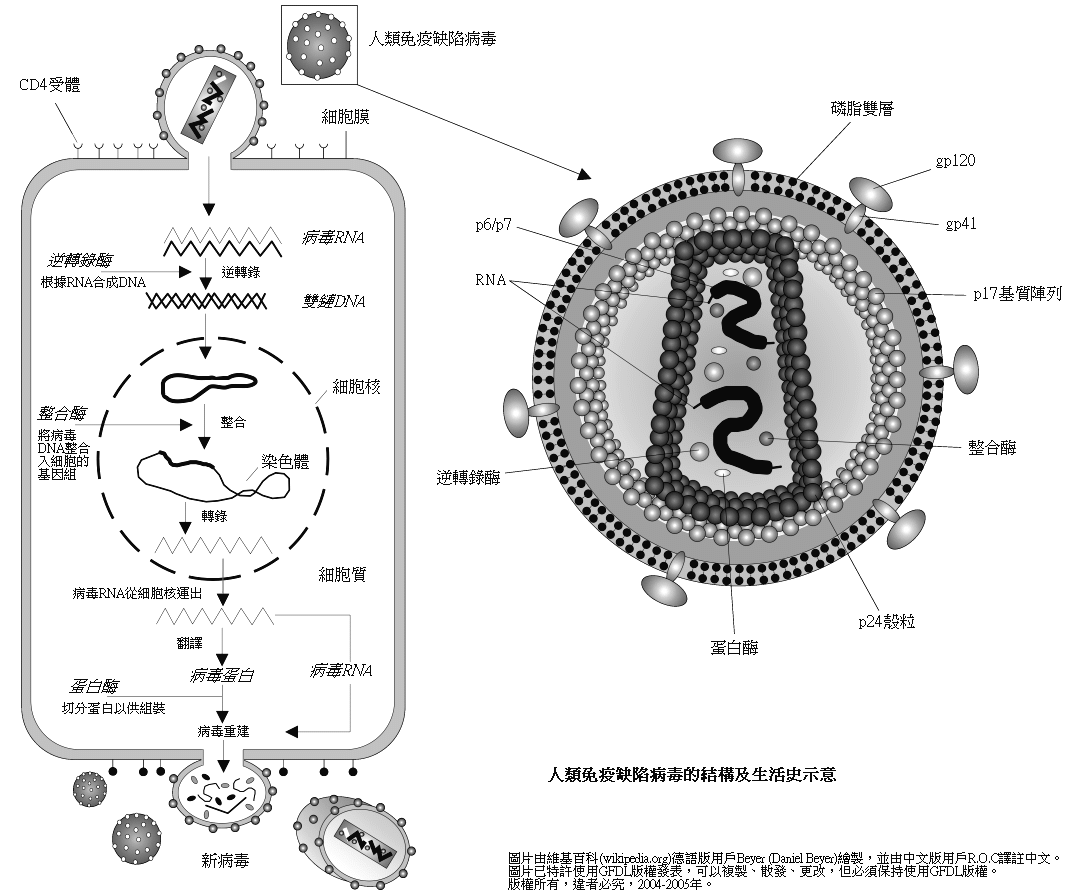
HIV structure
 The
The epitope
An epitope, also known as antigenic determinant, is the part of an antigen that is recognized by the immune system, specifically by antibodies, B cells, or T cells. The epitope is the specific piece of the antigen to which an antibody binds. The p ...
s of the viral envelope are more variable than those of many other viruses. Furthermore, the functionally important epitopes of the gp120
Envelope glycoprotein GP120 (or gp120) is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1988. The 120 in its name comes from ...
protein are masked by glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not al ...
, trimerisation
In chemistry, a trimer (; ) is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often ...
and receptor-induced conformational changes making it difficult to block with neutralizing antibodies.
The ineffectiveness of previously developed vaccines primarily stems from two related factors:
* First, HIV is highly mutable. Because of the virus's ability to rapidly respond to selective pressures imposed by the immune system, the population of virus in an infected individual typically evolves so that it can evade the two major arms of the adaptive immune system; humoral (antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
-mediated) and cellular (mediated by T cells
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell re ...
) immunity.
* Second, HIV isolates are themselves highly variable. HIV can be categorized into multiple subtypes with a high degree of genetic divergence. Therefore, the immune responses raised by any vaccine need to be broad enough to account for this variability. Any vaccine that lacks this breadth is unlikely to be effective.
The difficulties in stimulating a reliable antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
response has led to the attempts to develop a vaccine that stimulates a response by cytotoxic T-lymphocyte
A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pa ...
s.
Another response to the challenge has been to create a single peptide that contains the least variable components of all the known HIV strains.
It had been observed that a few, but not all, HIV-infected individuals naturally produce broadly neutralizing antibodies (BNAbs) which keep the virus suppressed, and these people remain asymptomatic for decades. Since the 2010s a core candidate is VRC01 and similar BNAbs, as they have been found in multiple unrelated people. These antibodies mimic CD4 and compete for the conserved CD4 binding site. These antibodies all share a germline
In biology and genetics, the germline is the population of a multicellular organism's cells that pass on their genetic material to the progeny (offspring). In other words, they are the cells that form the egg, sperm and the fertilised egg. They ...
origin in the VH chain, where only a few human allele
An allele (, ; ; modern formation from Greek ἄλλος ''állos'', "other") is a variation of the same sequence of nucleotides at the same place on a long DNA molecule, as described in leading textbooks on genetics and evolution.
::"The chro ...
s of the IVIG1-2 gene are able to produce such an antibody.
Animal model
animal model
An animal model (short for animal disease model) is a living, non-human, often genetic-engineered animal used during the research and investigation of human disease, for the purpose of better understanding the disease process without the risk of ha ...
for vaccine research is the monkey, often the macaque
The macaques () constitute a genus (''Macaca'') of gregarious Old World monkeys of the subfamily Cercopithecinae. The 23 species of macaques inhabit ranges throughout Asia, North Africa, and (in one instance) Gibraltar. Macaques are principally ...
. Monkeys can be infected with SIV or the chimeric SHIV for research purposes. However, the well-proven route of trying to induce neutralizing antibodies by vaccination has stalled because of the great difficulty in stimulating antibodies that neutralise heterologous primary HIV isolates. Some vaccines based on the virus envelope have protected chimpanzees or macaques from homologous virus challenge, but in clinical trials, humans who were immunised with similar constructs became infected after later exposure to HIV-1.
There are some differences between SIV and HIV that may introduce challenges in the use of an animal model. The animal model can be extremely useful but at times controversial.
There is a new animal model strongly resembling that of HIV in humans. Generalized immune activation as a direct result of activated CD4+ T cell killing - performed in mice allows new ways of testing HIV behaviour.
NIAID
The National Institute of Allergy and Infectious Diseases (NIAID, ) is one of the 27 institutes and centers that make up the National Institutes of Health (NIH), an agency of the United States Department of Health and Human Services (HHS). NIAID's ...
-funded SIV research has shown that challenging monkeys with a cytomegalovirus
''Cytomegalovirus'' (''CMV'') (from ''cyto-'' 'cell' via Greek - 'container' + 'big, megalo-' + -''virus'' via Latin 'poison') is a genus of viruses in the order ''Herpesvirales'', in the family ''Herpesviridae'', in the subfamily ''Betaherpe ...
(CMV)-based SIV vaccine results in containment of virus. Typically, virus replication and dissemination occurs within days after infection, whereas vaccine-induced T cell activation and recruitment to sites of viral replication take weeks. Researchers hypothesized that vaccines designed to maintain activated effector memory T cells might impair viral replication at its earliest stage.
Specific vaccines may also need specialized animal models. For example, vaccines designed to produce VRC01-type antibodies require human-like VH alleles to be present. For organisms like mice, the human allele must be inserted into their genome to produce a useful mimic.
Clinical trials
Several vaccine candidates are in varying phases ofclinical trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietar ...
s.
Phase I
Most initial approaches have focused on theHIV envelope
''Env'' is a viral gene that encodes the protein forming the viral envelope. The expression of the ''env'' gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
Analysis of the structure ...
protein. At least thirteen different gp120
Envelope glycoprotein GP120 (or gp120) is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1988. The 120 in its name comes from ...
and gp160
''Env'' is a viral gene that encodes the protein forming the viral envelope. The expression of the ''env'' gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
Analysis of the structure ...
envelope candidates have been evaluated, in the US predominantly through the AIDS Vaccine Evaluation Group. Most research focused on gp120 rather than gp41/gp160, as the latter is generally more difficult to produce and did not initially offer any clear advantage over gp120 forms. Overall, they have been safe and immunogenic in diverse populations, have induced neutralizing antibody in nearly 100% recipients, but rarely induced CD8+ cytotoxic T lymphocytes (CTL). Mammalian derived envelope preparations have been better inducers of neutralizing antibody than candidates produced in yeast and bacteria. Although the vaccination process involved many repeated "booster
Booster may refer to:
Amusement rides
* Booster (Fabbri ride), a pendulum ride
* Booster (HUSS ride), an evolution of the Breakdance ride
* Booster (KMG ride), a pendulum ride
Arts, entertainment, and media Fictional characters
*Booster, a char ...
" injections, it was challenging to induce and maintain the high anti-gp120 antibody titer
Titer (American English) or titre (British English) is a way of expressing concentration. Titer testing employs serial dilution to obtain approximate quantitative information from an analytical procedure that inherently only evaluates as positiv ...
s necessary to have any hope of neutralizing an HIV exposure.
The availability of several recombinant canarypox
Canarypox virus (CNPV) is an ''Avipoxvirus'' and etiologic agent of canarypox, a disease of wild and captive birds that can cause significant losses. Canarypox can enter human cells, but it cannot survive and multiply in human cells. There is a ...
vectors has provided interesting results that may prove to be generalizable to other viral vector
Viral vectors are tools commonly used by molecular biologists to deliver genetic material into cells. This process can be performed inside a living organism (''in vivo'') or in cell culture (''in vitro''). Viruses have evolved specialized molecul ...
s. Increasing the complexity of the canarypox vectors by including more genes/epitopes has increased the percent of volunteers that have detectable CTL to a greater extent than did increase the dose of the viral vector. CTLs from volunteers were able to kill peripheral blood mononuclear cells infected with primary isolates of HIV, suggesting that induced CTLs could have biological significance. Besides, cells from at least some volunteers were able to kill cells infected with HIV from other clades, though the pattern of recognition was not uniform among volunteers. The canarypox vector is the first candidate HIV vaccine that has induced cross-clade functional CTL responses. The first phase I trial of the candidate vaccine in Africa was launched early in 1999 with Ugandan volunteers. The study determined the extent to which Ugandan volunteers have CTL that are active against the subtypes of HIV prevalent in Uganda, A and D. In 2015, a Phase I trial called HVTN 100 in South Africa tested the combination of a canarypox vector ALVAC and a gp120 protein adapted for the subtype C HIV common in sub-Saharan Africa, with the MF59 adjuvant. Those who received the vaccine regimen produced strong immune responses early on and the regimen was safe.
Other strategies that have progressed to phase I trials in uninfected persons include peptides, lipopeptide
A lipopeptide is a molecule consisting of a lipid connected to a peptide. They are able to self-assemble into different structures. Many bacteria produced these molecules as a part of their metabolism, especially those of the genus ''Bacillus'', ' ...
s, DNA, an attenuated ''Salmonella
''Salmonella'' is a genus of rod-shaped (bacillus) Gram-negative bacteria of the family Enterobacteriaceae. The two species of ''Salmonella'' are ''Salmonella enterica'' and ''Salmonella bongori''. ''S. enterica'' is the type species and is fur ...
'' vector, p24, etc. Specifically, candidate vaccines that induce one or more of the following are being sought:
* neutralizing antibodies
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic.
Neutralizing antibod ...
active against a broad range of HIV primary isolates;
* cytotoxic T cell responses in a vast majority of recipients;
* strong mucosal immune response
An immune response is a reaction which occurs within an organism for the purpose of defending against foreign invaders. These invaders include a wide variety of different microorganisms including viruses, bacteria, parasites, and fungi which could ...
s.
In 2011, researchers in National Biotech Centre in Madrid
Madrid ( , ) is the capital and most populous city of Spain. The city has almost 3.4 million inhabitants and a metropolitan area population of approximately 6.7 million. It is the second-largest city in the European Union (EU), and ...
unveiled data from the Phase I clinical trial of their new vaccine, MVA-B MVA-B, or Modified Vaccinia Ankara B, is a HIV vaccine created to give immune resistance to infection by the human immunodeficiency virus. It was developed by a team of Spanish researchers at the Spanish National Research Council's Biotechnology Nat ...
. The vaccine induced an immunological response in 92% of the healthy subjects.
In 2016, results were published of the first Phase I human clinical trial of a killed whole-HIV-1 vaccine, SAV001 SAV001-H is the first candidate preventive HIV vaccine using a killed or "dead" version of the HIV-1 virus.
The vaccine was developed by Dr. Chil-Yong Kang and his research team at Western University’s Schulich School of Medicine & Dentistry in C ...
. HIV used in the vaccine was chemically and physically deadened through radiation. The trial, conducted in Canada in 2012, demonstrated a good safety profile and elicited antibodies to HIV-1. According to Dr. Chil-Yong Kang of Western University's Schulich School of Medicine & Dentistry
The Schulich School of Medicine & Dentistry is the combined medical school and dental school of the University of Western Ontario, one of 17 medical schools in Canada and one of six in Ontario.
History
The medical school at the University of ...
in Canada, the developer of this vaccine, antibodies against gp120 and p24 increased to 8-fold and 64-fold, respectively after vaccination.
The VRC01 line of research produced an "eOD-GT8" antigen which specifically exposes the CD4 binding site for immunization, refined over time to expose less of the other sites. As it turns out that most (but not all) humans do have the required alleles, the problem shifted to the method of delivery. In 2021, after promising results in tests with mice and primates, scientists announced that they plan to conduct a Phase 1 trial of an mRNA vaccine
An mRNA vaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. The vaccine delivers molecules of antigen-encoding mRNA into immune cells, which use the designed mRNA as a blueprin ...
against HIV, via their 'env–gag VLP mRNA platform' containing eOD-GT8, further developed vaccine is confirmed safe and effective. On January 17, 2022 IAVI and Moderna
Moderna, Inc. ( ) is an American pharmaceutical and biotechnology company based in Cambridge, Massachusetts that focuses on RNA therapeutics, primarily mRNA vaccines. These vaccines use a copy of a molecule called messenger RNA (mRNA) to produ ...
launched a phase I trial of a HIV vaccine with mRNA technology. On March 14, 2022 the National Institutes of Health (www.nih.gov) published: "NIH launches clinical trial of three mRNA HIV vaccines". The phase one trial is expected to conclude July 2023.
Phase II
''Preventive HIV vaccines'' * A recombinant Adenovirus-5 HIV vaccine (called V520) was tested in two Phase 2b studies, Phambili and STEP. On December 13, 2004, recruitment began for theSTEP study The STEP Study was a Phase IIb clinical trial intended to study the efficacy of an experimental HIV vaccine based on a human adenovirus 5 (HAdV-5) vector. The study was conducted in North and South America, the Caribbean, and Australia. A related s ...
, a 3,000-participant phase II clinical trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases ...
of a novel HIV vaccine, at sites in North America, South America, the Caribbean and Australia. The trial was co-funded by the National Institute of Allergy and Infectious Diseases
The National Institute of Allergy and Infectious Diseases (NIAID, ) is one of the 27 institutes and centers that make up the National Institutes of Health (NIH), an agency of the United States Department of Health and Human Services (HHS). NIAID's ...
(NIAID), which is a division of the National Institutes of Health
The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late ...
(NIH), and the pharmaceutical company Merck & Co. Merck developed V520 to stimulate HIV-specific cellular immunity, which prompts the body to produce T cells that kill HIV-infected cells. In previous smaller trials, this vaccine was found to be safe, because of the lack of adverse effects on the participants. The vaccine showed induced cellular immune responses against HIV in more than half of volunteers. V520 contains a weakened adenovirus
Adenoviruses (members of the family ''Adenoviridae'') are medium-sized (90–100 nm), nonenveloped (without an outer lipid bilayer) viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from the ...
that serves as a carrier for three subtype B HIV genes (''gag,'' ''pol'' and ''nef''). Subtype B is the most prevalent HIV subtype in the regions of the study sites. Adenoviruses are among the main causes of upper respiratory tract ailments such as the common cold
The common cold or the cold is a viral infectious disease of the upper respiratory tract that primarily affects the respiratory mucosa of the nose, throat, sinuses, and larynx. Signs and symptoms may appear fewer than two days after exposu ...
. Because the vaccine contains only three HIV genes housed in a weakened adenovirus, study participants cannot become infected with HIV or get a respiratory infection from the vaccine. It was announced in September 2007 that the trial for V520 would be stopped after it determined that vaccination with V520 appeared associated with an increased risk of HIV infection in some recipients. The foremost issue facing the recombinant adenovirus that was used is the high prevalence of the adenovirus-specific antibodies as a result of prior exposure to adenovirus. Adenovirus vectors and many other viral vectors
Viral vectors are tools commonly used by molecular biologists to deliver genetic material into cells. This process can be performed inside a living organism (''in vivo'') or in cell culture (''in vitro''). Viruses have evolved specialized molecul ...
currently used in HIV vaccines will induce a rapid memory immune response against the vector. This results in an impediment to the development of a T cell response against the inserted antigen (HIV antigens) The results of the trial prompted the reexamination of vaccine development strategies.
* HVTN 505, a Phase IIb study, was launched in 2009 but halted in 2013 due to meeting requirements of futility.
* Potential broadly neutralizing antibodies have been cloned in the laboratory (monoclonal antibodies) and are being tested in passive vaccination clinical trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietar ...
s. In May 2016, there was the launch of the Antibody Mediated Prevention (AMP) trials (HVTN 703 and HVTN 704), the first phase IIb trials of a monoclonal antibody for HIV prevention. HVTN 703 and HVTN 704 found that the VRC01 monoclonal antibody, which targets the CD4 binding site, was not able to prevent HIV acquisition.
* In 2017, Janssen and the HVTN launched the phase IIb trial called HVTN 705/Imbokodo, testing the mosaic vector vaccine Ad26.Mos4.HIV and the aluminum phosphate-adjuvanted Clade C gp140 vaccines which are designed to prevent infection of all HIV subtypes around the world. In 2021 the NIH
The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late ...
announced that the Imbokodo Phase 2b study did not provide statistically significant reduction in HIV infection.
* In 2019, Terevac-VIH, a vaccine from Cuba, was determined to have passed the first stage of clinical trials after two years and move to the second stage of development.
''Therapeutic HIV vaccines''
Biosantech developed a therapeutic vaccine called Tat Oyi, which targets the tat protein of HIV. It was tested in France in a double-blind Phase I/II trial with 48 HIV-positive patients who had reached viral suppression on Highly Active Antiretroviral Therapy
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple ...
and then stopped antiretrovirals after getting the intradermal Tat Oyi vaccine.
Phase III
''Preventive HIV vaccines'' There have been no passive preventive HIV vaccines to reach Phase III yet, but some active preventive HIV vaccine candidates have entered Phase III. * In February 2003,VaxGen
VaxGen was a biopharmaceutical company based in the San Francisco Bay Area.
Founded in 1995 and based in South San Francisco, California, the company was engaged in the development of vaccines that immunize against infectious disease, notably AI ...
announced that their AIDSVAX B/E vaccine was a failure in North America
North America is a continent in the Northern Hemisphere and almost entirely within the Western Hemisphere. It is bordered to the north by the Arctic Ocean, to the east by the Atlantic Ocean, to the southeast by South America and the Car ...
as there was not a statistically significant reduction of HIV infection within the study population.
* AIDSVAX B/E was a component, along with ALVAC, of the RV 144 RV 144, or the Thai trial, was an HIV vaccine clinical trial that was conducted in Thailand between 2003 and 2006. It used a combination of two HIV vaccines that had each failed in earlier trials. Participants were vaccinated over the course of 24 w ...
vaccine trial in Thailand that showed partial efficacy in preventing HIV. The AIDSVAX B/E and ALVAC vaccines targeted the gp120
Envelope glycoprotein GP120 (or gp120) is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1988. The 120 in its name comes from ...
part of the HIV envelope. The study involved 16,395 participants who did not have HIV infection, 8197 of whom were given treatment consisting of two experimental vaccines targeting HIV types B and E that are prevalent in Thailand, while 8198 were given a placebo. The participants were tested for HIV every six months for three years. After three years, the vaccine group had HIV infection rates reduced by about 30% compared with those in the placebo group. However, after taking into account the seven people who already had HIV before getting vaccinated (two in the placebo group, five in the vaccine group) the difference was 26%. It was discovered that participants receiving vaccines in the RV 144 trial who produced IgG
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG a ...
antibodies against the V2 loop
V, or v, is the twenty-second and fifth-to-last letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''vee'' (pronounced ), plural ...
of the HIV outer envelope were 43% less likely to become infected than those who did not, while IgA Iga may refer to:
Arts and entertainment
* Ambush at Iga Pass, a 1958 Japanese film
* Iga no Kagemaru, Japanese manga series
* Iga, a set of characters from the Japanese novel '' The Kouga Ninja Scrolls''
Biology
* ''Iga'' (beetle), a gen ...
production was associated with a 54% greater risk of infection than those who did not produce the antibodies (but not worse than placebo). Viruses collected from vaccinated participants had mutations in the V2 region. Tests of a vaccine for SIV in monkeys found greater resistance to SIV in animals producing antibodies against this region. Therefore, further research is expected to focus on creating vaccines designed to provoke an IgG reaction against the V2 loop.
* In 2020, the phase IIb-III trial /"Uhambo" found that ALVAC/gp120/MF59 vaccinations were safe, and caused no harm, but had no efficacy in HIV prevention in South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring countri ...
. Vaccinations with the Uhambo vaccine regimen began late 2016 and stopped early 2020.
* In 2020, the Ad26.Mos4.HIV plus adjuvanted clade C gp140 vaccine regimen entered a phase III trial called HVTN 706/"Mosaico". The regimen is a combination of an adenovirus vector vaccine engineered against multiple global strains of HIV, and a protein vaccine.
''Therapeutic HIV vaccines''
No therapeutic HIV vaccine candidates have reached phase 3 testing yet.
Economics
A July 2012 report of the HIV Vaccines & Microbicides Resource Tracking Working Group estimates that $845 million was invested in HIV vaccine research in 2011. Economic issues with developing an HIV vaccine include the need for advance purchase commitment (oradvance market commitments
An advance market commitment (AMC) is a binding contract, typically offered by a government or other financial entity, used to guarantee a viable market for a product once it is successfully developed. Generally AMCs are used in circumstances wher ...
) because after an AIDS vaccine has been developed, governments and NGOs may be able to bid the price down to marginal cost
In economics, the marginal cost is the change in the total cost that arises when the quantity produced is incremented, the cost of producing additional quantity. In some contexts, it refers to an increment of one unit of output, and in others it r ...
.
Classification of possible vaccines
Theoretically, any possible HIV vaccine must inhibit or stop the HIV virion replication cycle. The targets of a vaccine could be the following stages of the HIV virion cycle: * Stage I. Free state * Stage II. Attachment * Stage III. Penetration * Stage IV. Uncoating * Stage V. Replication * Stage VI. Assembling * Stage VII. Releasing Therefore, the following list comprises the current possible approaches for an HIV vaccine:Filtering virions from blood (Stage I)
* Biological, chemical and/or physical approaches for removing the HIV virions from the blood.Approaches to catching the virion (Stage I-III, VI, VII)
*Phagocytosis
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is ...
of the HIV virions.
* Chemical or organically based capture (creation of any skin or additional membrane around the virion) of HIV virions
* Chemical or organic attachments to the virion
Approaches to destroying or damaging the virion or its parts (Stage I-VII)
Here, "damage" means inhibiting or stopping the ability of virion to process any of the ''Phase II-VII''. Here are the different classification of methods: * By nature of method: ** Physical methods (''Stage I-VII'') ** Chemical and biological methods (''Stage I-VII'') * By damaging target of the HIV virion structure: ** Damaging the Docking Glycoprotein gp120 (''Stage I-III, VI, VII'') ** Damaging the Transmembrane Glycoprotein gp41 (''Stage I-III, VI, VII'') ** Damaging the virion matrix (''Stage I-III, VI, VII'') ** Damaging the virion Capsid (''Stage I-III, VI, VII'') ** Damaging the Reverse Transcriptase (''Stage I-VII'') ** Damaging the RNA (''Stage I-VII'')Blocking replication (Stage V)
* Insertion into blood chemical or organic compounds which binds to thegp120
Envelope glycoprotein GP120 (or gp120) is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1988. The 120 in its name comes from ...
. Hypothetically, it can be pieces of the CD4
In molecular biology, CD4 (cluster of differentiation 4) is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as T helper cells, monocytes, macrophages, and dendritic ...
cell membranes with receptors. Any chemical and organic alternative (with the ability to bind the gp120) of these receptors also can be used.
* Insertion into blood chemical or organic compounds which binds to the receptors of the CD4 cells.
Biological, chemical or physical approaches to inhibit the process of phases
* Biological, chemical or physical approach to inhibit the ''Attachment, Penetration, Uncoating, Integration, Replication, Assembling and/or Releasing.''Inhibiting the functionality of infected cells (Stage VI-VII)
Inhibiting the life functions of infected cells: * Inhibiting the metabolism of infected cells * Inhibiting the energy exchange of infected cellsFuture work
There have been reports that HIV patients coinfected with ''GB virus C
GB virus C (GBV-C), formerly known as hepatitis G virus (HGV) and also known as human pegivirus – HPgV is a virus in the family ''Flaviviridae'' and a member of the ''Pegivirus'', is known to infect humans, but is not known to cause human dise ...
'' (GBV-C), also called hepatitis G virus, can survive longer than those without GBV-C, but the patients may be different in other ways. GBV-C is potentially useful in the future development of an HIV vaccine.
Live attenuated vaccines are highly successful against polio, rotavirus and measles, but have not been tested against HIV in humans. Reversion to live virus has been a theoretical safety concern that has to date prevented clinical development of a live attenuated HIV-1 vaccine. Scientists are researching novel strategies to develop a non-virulent live attenuated HIV-1 vaccine. For example, a genetically modified form of HIV has been created in which the virus's codons
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
(a sequence of three nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules w ...
that form genetic code) are manipulated to rely on an unnatural amino acid for proper protein translation, which allows it to replicate. Because this amino acid is foreign to the human body, the virus cannot reproduce.
See also
*Cabotegravir
Cabotegravir, sold under the brand name Vocabria among others, is a antiretroviral medication used for the treatment of HIV/AIDS. It is available in the form of tablets and as an intramuscular injection, as well as in an injectable Cabotegravir ...
* COVID-19 vaccine
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 ( COVID19).
Prior to the COVID19 pandemic, an e ...
* HIV Vaccine Trials Network
The HIV Vaccine Trials Network (HVTN) is a non-profit organization which connects physicians and scientists with activists and community educators for the purpose of conducting clinical trials seeking a safe and effective HIV vaccine. Collaborativ ...
* World AIDS Vaccine Day {{No footnotes, date=March 2009
World AIDS Vaccine Day, also known as HIV Vaccine Awareness Day, is observed annually on May 18. HIV vaccine advocates mark the day by promoting the continued urgent need for a vaccine to prevent HIV infection and A ...
References
External links
Vaccine Research Center (VRC)
Information concerning Preventive HIV vaccine research studies
NIAID HIV vaccine site
(
DAIDS
The Division of Acquired Immunodeficiency Syndrome (DAIDS) is a division of the National Institute of Allergy and Infectious Diseases, which is part of the National Institutes of Health. It was formed in 1986 as a part of the initiative to address ...
)
Global Alliance for Vaccines and Immunization (GAVI)
International AIDS Vaccine Initiative (IAVI)
AIDS Vaccine Advocacy Coalition (AVAC)
U.S. Military HIV Research Program (MHRP)
HIV.gov - The U.S. Federal Domestic HIV/AIDS Resource
HIVtest.org - Find an HIV testing site near you
- The New York Times, March 8, 2019
Treatment Action Group
{{Authority control Prevention of HIV/AIDS Hypothetical technology