α-synuclein on:
[Wikipedia]
[Google]
[Amazon]
Alpha-synuclein (aSyn) is a
 Alpha synuclein, having no single, well-defined tertiary structure, is an intrinsically disordered protein, with a pI value of 4.7, which, under certain pathological conditions, can misfold in a way that exposes its core hydrophobic residues to the intracellular milieu, thus providing the opportunity for
Alpha synuclein, having no single, well-defined tertiary structure, is an intrinsically disordered protein, with a pI value of 4.7, which, under certain pathological conditions, can misfold in a way that exposes its core hydrophobic residues to the intracellular milieu, thus providing the opportunity for 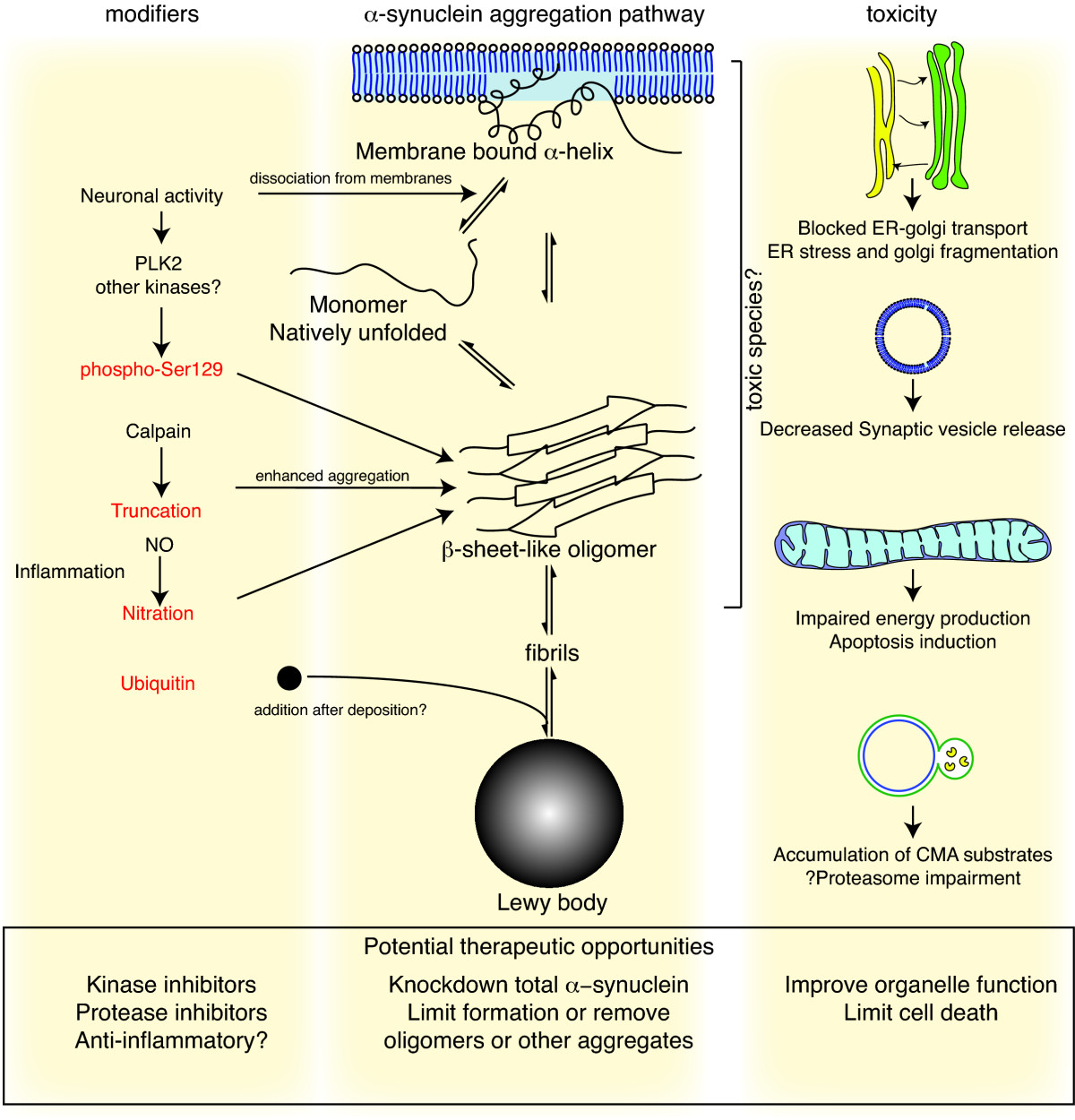 Self-replicating "prion-like" amyloid assemblies of alpha-synuclein have been described that are invisible to the amyloid dye Thioflavin T and that can acutely spread in neurons in vitro and in vivo.
Self-replicating "prion-like" amyloid assemblies of alpha-synuclein have been described that are invisible to the amyloid dye Thioflavin T and that can acutely spread in neurons in vitro and in vivo.
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
that in humans is encoded by the ''SNCA'' gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
. It is a neuronal protein involved in the regulation of synaptic vesicle trafficking and the release of neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a Chemical synapse, synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.
Neurotra ...
s.
Alpha-synuclein is abundant in the brain, with smaller amounts present in the heart, muscles, and other tissues. Within the brain, it is primarily localized to the axon terminal
Axon terminals (also called terminal boutons, synaptic boutons, end-feet, or presynaptic terminals) are distal terminations of the branches of an axon. An axon, also called a nerve fiber, is a long, slender projection of a Neuron, nerve cell tha ...
s of presynaptic neuron
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous syste ...
s. There, it interacts with phospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s and other proteins. Presynaptic terminals release neurotransmitters from specialized compartments called synaptic vesicle
In a neuron, synaptic vesicles (or neurotransmitter vesicles) store various neurotransmitters that are exocytosis, released at the chemical synapse, synapse. The release is regulated by a voltage-dependent calcium channel. Vesicle (biology), Ves ...
s, a process essential for neuronal communication and normal brain function.
In Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
and related synucleinopathies, abnormal, insoluble forms of alpha-synuclein accumulate within neurons as inclusions known as Lewy bodies.
Mutations in the ''SNCA'' gene are linked to familial forms of Parkinson's disease. During the process of seeded nucleation, alpha-synuclein adopts a cross-beta sheet structure characteristic of amyloid
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human ...
fibrils.
The human alpha-synuclein protein consists of 140 amino acids. A fragment of alpha-synuclein, known as the non-amyloid beta
Amyloid beta (Aβ, Abeta or beta-amyloid) denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease. The peptides derive from the amyloid-beta precursor prot ...
component (NAC) of Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems wit ...
amyloid
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human ...
, was initially isolated from an amyloid-rich brain fraction and shown to derive from a precursor protein named NACP. NACP was subsequently identified as the human homologue of synuclein from the electric ray genus ''Torpedo
A modern torpedo is an underwater ranged weapon launched above or below the water surface, self-propelled towards a target, with an explosive warhead designed to detonate either on contact with or in proximity to the target. Historically, such ...
'', leading to its renaming as human alpha-synuclein.
Tissue expression
Alpha-synuclein is asynuclein
Synucleins are a family of soluble proteins common to vertebrates, primarily expressed in neural tissue and in certain tumors.
The name is a blend of the words "synapse" and "nucleus", as it was first found in the synapses in the electromotor n ...
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
primarily found in neural tissue, making up as much as one percent of all proteins in the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
of brain cell
Brain cells make up the functional tissue of the brain. The rest of the brain tissue is the structural stroma that includes connective tissue such as the meninges, blood vessels, and ducts. The two main types of cells in the brain are neurons, ...
s. It is expressed highly in neurons within the frontal cortex
The frontal lobe is the largest of the four major lobes of the brain in mammals, and is located at the front of each cerebral hemisphere (in front of the parietal lobe and the temporal lobe). It is parted from the parietal lobe by a groove betw ...
, hippocampus
The hippocampus (: hippocampi; via Latin from Ancient Greek, Greek , 'seahorse'), also hippocampus proper, is a major component of the brain of humans and many other vertebrates. In the human brain the hippocampus, the dentate gyrus, and the ...
, striatum
The striatum (: striata) or corpus striatum is a cluster of interconnected nuclei that make up the largest structure of the subcortical basal ganglia. The striatum is a critical component of the motor and reward systems; receives glutamat ...
, and olfactory bulb
The olfactory bulb (Latin: ''bulbus olfactorius'') is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OF ...
, but can also be found in the non-neuronal glial cells
Glia, also called glial cells (gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (the brain and the spinal cord) and in the peripheral nervous system that do not produce electrical impulses. The neuroglia make up ...
. In melanocyte
Melanocytes are melanin-producing neural-crest, neural crest-derived cell (biology), cells located in the bottom layer (the stratum basale) of the skin's epidermis (skin), epidermis, the middle layer of the eye (the uvea),
the inner ear,
vagina ...
s, SNCA protein expression may be regulated by microphthalmia-associated transcription factor
Microphthalmia-associated transcription factor also known as class E basic helix-loop-helix protein 32 or bHLHe32 is a protein that in humans is encoded by the ''MITF'' gene.
MITF is a basic helix-loop-helix leucine zipper transcription factor ...
(MITF).
It has been established that alpha-synuclein is extensively localized in the nucleus of mammalian brain neurons, suggesting a role of alpha-synuclein in the nucleus. Synuclein is however found predominantly in the presynaptic
In the nervous system, a synapse is a structure that allows a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or a target effector cell. Synapses can be classified as either chemical or electrical, depending o ...
termini, in both free or membrane-bound forms, with roughly 15% of synuclein being membrane-bound at any moment in neurons.
It has also been shown that alpha-synuclein is localized in neuronal mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
. Alpha-synuclein is highly expressed in the mitochondria in olfactory bulb
The olfactory bulb (Latin: ''bulbus olfactorius'') is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OF ...
, hippocampus, striatum and thalamus, where the cytosolic alpha-synuclein is also rich. However, the cerebral cortex and cerebellum are
two exceptions, which contain rich cytosolic alpha-synuclein but very low levels of mitochondrial alpha-synuclein. It has been shown that alpha-synuclein is localized in the inner membrane of mitochondria, and that the inhibitory effect of alpha-synuclein on complex I
Respiratory complex I, (also known as NADH:ubiquinone oxidoreductase, Type I NADH dehydrogenase and mitochondrial complex I) is the first large protein complex of the respiratory chains of many organisms from bacteria to humans. It catalyzes th ...
activity of the mitochondrial respiratory chain is dose-dependent. Thus, it is suggested that alpha-synuclein in mitochondria is differentially expressed in different brain regions and the background levels of mitochondrial alpha-synuclein may be a potential factor affecting mitochondrial function and predisposing some neurons to degeneration.
At least three isoforms of synuclein are produced through alternative splicing
Alternative splicing, alternative RNA splicing, or differential splicing, is an alternative RNA splicing, splicing process during gene expression that allows a single gene to produce different splice variants. For example, some exons of a gene ma ...
. The majority form of the protein, and the one most investigated, is the full-length protein of 140 amino acids. Other isoforms are alpha-synuclein-126, which lacks residues 41-54 due to loss of exon 3; and alpha-synuclein-112, which lacks residues 103-130 due to loss of exon 5.
In the enteric nervous system (ENS)
First characterisations of aSyn aggregates in the ENS of PD patients has been performed on autopsied specimens in the late 1980s. It is yet unknown if the microbiome changes associated with PD are consequential to the illness process or main pathophysiology, or both. Individuals diagnosed with various synucleinopathies often display constipation and other GI dysfunctions years prior to the onset of movement dysfunction. Alpha synuclein potentially connects the gut-brain axis inParkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
patients. Common inherited Parkinson disease is associated with mutations in the alpha-synuclein (SNCA) gene. In the process of seeded nucleation, alpha-synuclein acquires a cross-sheet structure similar to other amyloids.
The ''Enterobacteriaceae
Enterobacteriaceae is a large family (biology), family of Gram-negative bacteria. It includes over 30 genera and more than 100 species. Its classification above the level of Family (taxonomy), family is still a subject of debate, but one class ...
'', which are quite common in the human gut, can create curli, which are functional amyloid proteins. The unfolded amyloid
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human ...
CsgA, which is secreted by bacteria and later aggregates extracellularly to create biofilms, mediates adherence to epithelial cells, and aids in bacteriophage defense, forms the curli fibers. Oral injection of curli-producing bacteria can also boost formation and aggregation of the amyloid protein Syn in old rats and nematode
The nematodes ( or ; ; ), roundworms or eelworms constitute the phylum Nematoda. Species in the phylum inhabit a broad range of environments. Most species are free-living, feeding on microorganisms, but many are parasitic. Parasitic worms (h ...
s. Host inflammation responses in the intestinal tract and periphery are modulated by curli exposure. Studies in biochemistry show that endogenous, bacterial chaperones of curli are capable of briefly interacting with Syn and controlling its aggregation.
The clinical and pathological findings support the hypothesis that aSyn disease in PD occurs via a gut-brain pathway. For early diagnosis and early management in the phase of creation and propagation of aSyn, it is therefore of utmost importance to identify pathogenic aSyn in the digestive system, for example, by gastrointestinal tract (GIT) biopsies.
According to a growing body of research, intestinal dysbiosis may be a major factor in the development of Parkinson's disease by encouraging intestinal permeability, gastrointestinal inflammation, and the aggregation and spread of asyn.
Not just the CNS but other peripheral tissues, such as the GIT, have physiological aSyn expression as well as its phosphorylated variants. As suggested by Borghammer and Van Den Berge (2019), one approach is to recognise the possibility of PD subtypes with various aSyn propagation methods, including either a peripheral nervous system (PNS)-first or a CNS-first route.
While the GI tract has been linked to other neurological disorders such autism spectrum disorder
Autism, also known as autism spectrum disorder (ASD), is a neurodevelopmental disorder characterized by differences or difficulties in social communication and interaction, a preference for predictability and routine, sensory processing di ...
, depression, anxiety
Anxiety is an emotion characterised by an unpleasant state of inner wikt:turmoil, turmoil and includes feelings of dread over Anticipation, anticipated events. Anxiety is different from fear in that fear is defined as the emotional response ...
, and Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems wit ...
, protein aggregation and/or inflammation in the gut represent a new topic of investigation in synucleinopathies.
Structure
Alpha-synuclein in solution is considered to be an intrinsically disordered protein, i.e. it lacks a single stable 3D structure. As of 2014, an increasing number of reports suggest, however, the presence of partial structures or mostly structured oligomeric states in the solution structure of alpha-synuclein even in the absence of lipids. This trend is also supported by a large number of single molecule (optical tweezers
Optical tweezers (originally called single-beam gradient force trap) are scientific instruments that use a highly focused laser beam to hold and move microscopic and sub-microscopic objects like atoms, nanoparticles and droplets, in a manner simil ...
) measurements on single copies of monomeric alpha-synuclein as well as covalently enforced dimers or tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula ...
s of alpha-synuclein.
Alpha-synuclein is specifically upregulated in a discrete population of presynaptic terminals of the brain during a period of acquisition-related synaptic rearrangement. It has been shown that alpha-synuclein significantly interacts with tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytosk ...
, and that alpha-synuclein may have activity as a potential microtubule-associated protein
In cell biology, microtubule-associated proteins (MAPs) are proteins that interact with the microtubules of the cellular cytoskeleton. MAPs are integral to the stability of the cell and its internal structures and the transport of components withi ...
, like tau
Tau (; uppercase Τ, lowercase τ or \boldsymbol\tau; ) is the nineteenth letter of the Greek alphabet, representing the voiceless alveolar plosive, voiceless dental or alveolar plosive . In the system of Greek numerals, it has a value of 300 ...
. Evidence suggests that alpha-synuclein functions as a molecular chaperone in the formation of SNARE
SNARE proteins – "Soluble NSF attachment protein, SNAP REceptors" – are a large protein family consisting of at least 24 members in yeasts and more than 60 members in mammalian and plant cells.
The primary role of SNARE proteins is to m ...
complexes. In particular, it simultaneously binds to phospholipids of the plasma membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
via its N-terminus domain and to synaptobrevin
Synaptobrevins (''synaptobrevin isotypes 1-2'') are small integral membrane proteins of secretory vesicles with molecular weight of 18 kilodalton (kDa) that are part of the vesicle-associated membrane protein (VAMP) family.
Synaptobre ...
-2 via its C-terminus domain, with increased importance during synaptic activity. Indeed, there is growing evidence that alpha-synuclein is involved in the functioning of the neuronal Golgi apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic Cell (biology), cells. Part of the endomembrane system in the cytoplasm, it protein targeting, packages proteins ...
and vesicle trafficking.
Apparently, alpha-synuclein is essential for normal development of the cognitive functions. Knock-out mice with the targeted inactivation of the expression of alpha-synuclein show impaired spatial learning and working memory.
Interaction with lipid membranes
Experimental evidence has been collected on the interaction of alpha-synuclein withmembrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
and its involvement with membrane composition and turnover. Yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom (biology), kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are est ...
genome screening has found that several genes that deal with lipid metabolism and mitochondrial fusion play a role in alpha-synuclein toxicity. Conversely, alpha-synuclein expression levels can affect the viscosity and the relative amount of fatty acids in the lipid bilayer.
Alpha-synuclein is known to directly bind to lipid membranes, associating with the negatively charged surfaces of phospholipids
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typi ...
. Alpha-synuclein forms an extended helical structure on small unilamellar vesicles. A preferential binding to small vesicles has been found. The binding of alpha-synuclein to lipid membranes has complex effects on the latter, altering the bilayer structure and leading to the formation of small vesicles. Alpha-synuclein has been shown to bend membranes of negatively charged phospholipid vesicles and form tubules from large lipid vesicles. Using cryo-EM
Cryogenic electron microscopy (cryo-EM) is a transmission electron microscopy technique applied to samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An ...
it was shown that these are micellar tubes of ~5-6 nm diameter. Alpha-synuclein has also been shown to form lipid disc-like particles similar to apolipoproteins. EPR studies have shown that the structure of alpha synuclein is dependent on the binding surface. The protein adopts a broken-helical conformation on lipoprotein particles while it forms an extended helical structure on lipid vesicles and membrane tubes. Studies have also suggested a possible antioxidant
Antioxidants are Chemical compound, compounds that inhibit Redox, oxidation, a chemical reaction that can produce Radical (chemistry), free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants ...
activity of alpha-synuclein in the membrane.
Membrane interaction of alpha-synuclein modulates or affects its rate of aggregation. The membrane-mediated modulation of aggregation is very similar to that observed for other amyloid proteins such as IAPP and abeta. Aggregated states of alpha-synuclein permeate the membrane of lipid vesicles. They are formed upon interaction with peroxidation-prone polyunsaturated fatty acid
In biochemistry and nutrition, a polyunsaturated fat is a fat that contains a polyunsaturated fatty acid (abbreviated PUFA), which is a subclass of fatty acid characterized by a backbone with two or more carbon–carbon double bonds.
Some polyunsa ...
s (PUFA) but not with monounsaturated fatty acids and the binding of lipid autoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organi ...
-promoting transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s such as iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
or copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
provokes oligomerization of alpha-synuclein. The aggregated alpha-synuclein has a specific activity for peroxidized lipids and induces lipid autoxidation in PUFA-rich membranes of both neurons and astrocytes, decreasing resistance to apoptosis. Lipid autoxidation is inhibited if the cells are pre-incubated with isotope-reinforced PUFAs (D-PUFA).
Function
Although the function of alpha-synuclein is not well understood, studies suggest that it plays a role in restricting the mobility of synaptic vesicles, consequently attenuating synaptic vesicle recycling and neurotransmitter release. An alternate view is that alpha-synuclein binds to VAMP2 (asynaptobrevin
Synaptobrevins (''synaptobrevin isotypes 1-2'') are small integral membrane proteins of secretory vesicles with molecular weight of 18 kilodalton (kDa) that are part of the vesicle-associated membrane protein (VAMP) family.
Synaptobre ...
) and stabilizes SNARE complexes; though recent studies indicate that alpha-synuclein–VAMP2 binding is critical for alpha-synuclein-mediated attenuation of synaptic vesicle recycling, connecting the two seemingly divergent views. It may also help regulate the release of dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. It is an amine synthesized ...
, a type of neurotransmitter that is critical for controlling the start and stop of voluntary and involuntary movements.
Alpha-synuclein modulates DNA repair
DNA repair is a collection of processes by which a cell (biology), cell identifies and corrects damage to the DNA molecules that encode its genome. A weakened capacity for DNA repair is a risk factor for the development of cancer. DNA is cons ...
processes, including repair of double-strand break
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. A weakened capacity for DNA repair is a risk factor for the development of cancer. DNA is constantly modified ...
s (DSBs). DNA damage
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. A weakened capacity for DNA repair is a risk factor for the development of cancer. DNA is constantly modified ...
response markers co-localize with alpha-synuclein to form discrete foci in human cells and mouse brain. Depletion of alpha-synuclein in human cells causes increased introduction of DNA DSBs after exposure to bleomycin
-13- (1''H''-imidazol-5-yl)methyl9-hydroxy-5- 1''R'')-1-hydroxyethyl8,10-dimethyl-4,7,12,15-tetraoxo-3,6,11,14-tetraazapentadec-1-yl}-2,4'-bi-1,3-thiazol-4-yl)carbonyl]amino}propyl)(dimethyl)sulfonium
, C=55 , H=84 , N=17 , O=21 , S=3
, SMI ...
and reduced ability to repair these DSBs. In addition, alpha-synuclein knockout mouse, knockout mice display a higher level of DSBs, and this problem can be alleviated by transgenic reintroduction of human alpha-synuclein. Alpha-synuclein promotes the DSB repair pathway referred to as non-homologous end joining
Non-homologous end joining (NHEJ) is a pathway that repairs double-strand breaks in DNA. It is called "non-homologous" because the break ends are directly ligated without the need for a homologous template, in contrast to homology directed repair ...
. The DNA repair function of alpha-synuclein appears to be compromised in Lewy body
Lewy bodies are the inclusion bodies – abnormal aggregations of protein – that develop inside neurons affected by Parkinson's disease (PD), the Lewy body dementias ( Parkinson's disease dementia and dementia with Lewy bodies (DL ...
inclusion bearing neurons, and this may trigger cell death.
Proneurogenic function of alpha-synuclein
In someneurodegenerative diseases
A neurodegenerative disease is caused by the progressive loss of neurons, in the process known as neurodegeneration. Neuronal damage may also ultimately result in their death. Neurodegenerative diseases include amyotrophic lateral sclerosis, mul ...
, alpha-synuclein produces insoluble inclusion bodies
Inclusion bodies are aggregates of specific types of protein found in neurons, and a number of tissue (biology), tissue cells including red blood cells, bacteria, viruses, and plants. Inclusion bodies of aggregations of multiple proteins are also ...
. These diseases, known as synucleinopathies, are connected with either higher levels of normal alpha-synuclein or its mutant variants. The normal physiological role of Snca, however, has not yet been thoroughly explained. In fact, physiological Snca has been demonstrated to have a neuroprotective impact by inhibiting apoptosis induced by several types of apoptotic stimuli, or by regulating the expression of proteins involved in apoptotic pathways.
Recently it has been demonstrated that up-regulation of alpha-synuclein in the dentate gyrus (a neurogenic niche where new neurons are generated throughout life) activates stem cells, in a model of premature neural aging. This model shows reduced expression of alpha-synuclein and reduced proliferation of stem cells, as is physiologically observed during aging. Exogenous alpha-synuclein in the dentate gyrus is able to rescue this defect. Moreover, alpha-synuclein also boosts the proliferation of dentate gyrus progenitor neural cells in wild-type young mice. Thus, alpha-synuclein represents an effector for neural stem and progenitor cell activation.
Similarly, alpha-synuclein has been found to be required to maintain stem cells of the SVZ (subventricular zone, i.e., another neurogenic niche) in a cycling state.
Sequence
Alpha-synucleinprimary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthe ...
is usually divided in three distinct domains:
* Residues 1-60: An amphipathic
In chemistry, an amphiphile (), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'', nonpolar) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic c ...
N-terminal region dominated by four 11-residue repeats including the consensus sequence
In molecular biology and bioinformatics, the consensus sequence (or canonical sequence) is the calculated sequence of most frequent residues, either nucleotide or amino acid, found at each position in a sequence alignment. It represents the result ...
KTKEGV. This sequence has a structural alpha helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the Protein secondary structure, secondary structure of proteins. It is al ...
propensity similar to apolipoproteins-binding domains. It is a highly conserved terminal that interacts with acidic lipid membranes, and all the discovered point mutations of the SNCA gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
are located within this terminal.
* Residues 61-95: A central hydrophobic region which includes the ''non-amyloid-β component'' (NAC) region, involved in protein aggregation. This domain is unique to alpha-synuclein among the synuclein family.
* Residues 96-140: a highly acidic and proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the p ...
-rich region which has no distinct structural propensity. This domain plays an important role in the function, solubility and interaction of alpha-synuclein with other protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s.
Autoproteolytic activity
The use of high-resolution ion-mobility mass spectrometry (IMS-MS) on HPLC-purified alpha-synuclein ''in vitro'' has shown alpha-synuclein to be autoproteolytic (self-proteolytic
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Protein degradation is a major regulatory mechanism of gene expression and contributes substantially to shaping mammalian proteomes. Uncatalysed, the hydrolysis o ...
), generating a variety of small molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
fragments upon incubation. The 14.46 kDa protein was found to generate numerous smaller fragments, including 12.16 kDa (amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
14–133) and 10.44 kDa (40–140) fragments formed through C- and N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
truncation and a 7.27 kDa C-terminal fragment (72–140). The 7.27 kDa fragment, which contains the majority of the NAC region, aggregated considerably faster than full-length alpha-synuclein. It is possible that these autoproteolytic products play a role as intermediates or cofactors in the aggregation of alpha-synuclein ''in vivo''.
Clinical significance
 Alpha synuclein, having no single, well-defined tertiary structure, is an intrinsically disordered protein, with a pI value of 4.7, which, under certain pathological conditions, can misfold in a way that exposes its core hydrophobic residues to the intracellular milieu, thus providing the opportunity for
Alpha synuclein, having no single, well-defined tertiary structure, is an intrinsically disordered protein, with a pI value of 4.7, which, under certain pathological conditions, can misfold in a way that exposes its core hydrophobic residues to the intracellular milieu, thus providing the opportunity for hydrophobic interactions
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and to be excluded by water. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar ...
to occur with a similar, equally exposed protein. This could lead to self assembly and subsequent aggregation into large, insoluble fibrils known as amyloids. The conversion of soluble alpha synuclein into highly ordered, cross-β sheet, fibrillar structures does not, as previously thought, follow a two-step mechanism, rather, occurs through a series of transient, soluble oligomeric
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomer, monomers.Quote: ''Oligomer molecule: A molecule of intermediate ...
intermediates. In 2011, two groups published their findings that unmutated α-synuclein forms a stably folded tetramer that resists aggregation, asserting that this folded tetramer represented the relevant in vivo structure in cells, thereby relieving alpha synuclein of its disordered status. Proponents of the tetramer hypothesis argued that in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, an ...
cross-linking in bacteria, primary neurons and human erythroleukemia cells confirmed the presence of labile, tetrameric species. However, despite numerous in-cell NMR reports demonstrating that alpha synuclein is indeed monomeric and disordered in intact E. coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly foun ...
cells, it is still a matter of debate in the field despite an ever growing mountain of conflicting reports. Nevertheless, alpha-synuclein aggregates to form insoluble fibrils in pathological conditions characterized by Lewy bodies, such as Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
, dementia with Lewy bodies
Dementia with Lewy bodies (DLB) is a type of dementia characterized by changes in sleep, behavior change (individual), behavior, cognition, movement, and dysautonomia, regulation of automatic bodily functions. Unlike some other dementias, mem ...
and multiple system atrophy
Multiple system atrophy (MSA) is a rare neurodegenerative disorder characterized by tremors, slow movement, muscle rigidity, postural instability (collectively known as parkinsonism), autonomic dysfunction and ataxia. This is caused by progr ...
. These disorders are known as synucleinopathies. In vitro models of synucleinopathies revealed that aggregation of alpha-synuclein may lead to various cellular disorders including microtubule impairment, synaptic and mitochondrial dysfunctions, oxidative stress as well as dysregulation of Calcium signaling, proteasomal and lysosomal pathway. Alpha-synuclein is the primary structural component of Lewy body fibrils. Occasionally, Lewy bodies contain tau protein
The tau proteins (abbreviated from tubulin associated unit) form a group of six highly soluble protein isoforms produced by alternative splicing from the gene ''MAPT'' (microtubule-associated protein tau). They have roles primarily in maintainin ...
; however, alpha-synuclein and tau constitute two distinctive subsets of filaments in the same inclusion bodies. Alpha-synuclein pathology is also found in both sporadic and familial cases with Alzheimer's disease.
The aggregation mechanism of alpha-synuclein is uncertain. There is evidence of a structured intermediate rich in beta structure that can be the precursor of aggregation and, ultimately, Lewy bodies. A single molecule study in 2008 suggests alpha-synuclein exists as a mix of unstructured, alpha-helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of ...
, and beta-sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common structural motif, motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone chain, backbon ...
-rich conformers in equilibrium. Mutations or buffer conditions known to improve aggregation strongly increase the population of the beta conformer, thus suggesting this could be a conformation related to pathogenic aggregation. One theory is that the majority of alpha-synuclein aggregates are located in the presynapse as smaller deposits which causes synaptic dysfunction. Among the strategies for treating synucleinopathies are compounds that inhibit aggregation of alpha-synuclein. It has been shown that the small molecule cuminaldehyde inhibits fibrillation of alpha-synuclein.
The Epstein-Barr virus has been implicated in these disorders.
In rare cases of familial forms of Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
, there is a mutation in the gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
coding for alpha-synuclein. Five point mutation
A point mutation is a genetic mutation where a single nucleotide base is changed, inserted or deleted from a DNA or RNA sequence of an organism's genome. Point mutations have a variety of effects on the downstream protein product—consequences ...
s have been identified thus far: A53T, A30P, E46K, H50Q, and G51D; however, in total, nineteen mutations in the SNCA gene have been associated with parkinsonism: A18T, A29S, A53E, A53V, E57A, V15A, T72M, L8I, V15D, M127I, P117S, M5T, G93A, E83Q, and A30G.
It has been reported that some mutations influence the initiation and amplification steps of the aggregation process. Genomic duplication and triplication of the gene appear to be a rare cause of Parkinson's disease in other lineages, although more common than point mutations. Hence certain mutations of alpha-synuclein may cause it to form amyloid-like fibrils that contribute to Parkinson's disease. Over-expression of human wild-type or A53T-mutant alpha-synuclein in primates drives deposition of alpha-synuclein in the ventral midbrain, degeneration of the dopaminergic system and impaired motor performance. Although the accumulation and aggregation of alpha-synuclein in most Parkinson's disease patients primarily result from posttranscriptional mechanisms, targeting its production remains a potential therapeutic approach. Research indicates that microRNA-7 and the naturally occurring small molecule quercetin
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor ...
can reduce alpha-synuclein levels under experimental conditions.
Certain sections of the alpha-synuclein protein may play a role in the tauopathies.
A prion
A prion () is a Proteinopathy, misfolded protein that induces misfolding in normal variants of the same protein, leading to cellular death. Prions are responsible for prion diseases, known as transmissible spongiform encephalopathy (TSEs), w ...
form of the protein alpha-synuclein may be a causal agent for the disease multiple system atrophy
Multiple system atrophy (MSA) is a rare neurodegenerative disorder characterized by tremors, slow movement, muscle rigidity, postural instability (collectively known as parkinsonism), autonomic dysfunction and ataxia. This is caused by progr ...
.
 Self-replicating "prion-like" amyloid assemblies of alpha-synuclein have been described that are invisible to the amyloid dye Thioflavin T and that can acutely spread in neurons in vitro and in vivo.
Self-replicating "prion-like" amyloid assemblies of alpha-synuclein have been described that are invisible to the amyloid dye Thioflavin T and that can acutely spread in neurons in vitro and in vivo.
Antibodies
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that caus ...
against alpha-synuclein have replaced antibodies against ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 19 ...
as the gold standard for immunostaining
In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by ...
of Lewy bodies. The central panel in the figure to the right shows the major pathway for protein aggregation. Monomeric α-synuclein is natively unfolded in solution but can also bind to membranes in an α-helical form. It seems likely that these two species exist in equilibrium within the cell, although this is unproven. From in vitro work, it is clear that unfolded monomer can aggregate first into small oligomeric species that can be stabilized by β-sheet-like interactions and then into higher molecular weight insoluble fibrils. In a cellular context, there is some evidence that the presence of lipids can promote oligomer formation: α-synuclein can also form annular, pore-like structures that interact with membranes. The deposition of α-synuclein into pathological structures such as Lewy bodies is probably a late event that occurs in some neurons. On the left hand side are some of the known modifiers of this process. Electrical activity in neurons changes the association of α-synuclein with vesicles and may also stimulate polo-like kinase 2 (PLK2), which has been shown to phosphorylate α-synuclein at Ser129. Other kinases have also been proposed to be involved. As well as phosphorylation, truncation through proteases such as calpains, and nitration, probably through nitric oxide (NO) or other reactive nitrogen species that are present during inflammation, all modify synuclein such that it has a higher tendency to aggregate. The addition of ubiquitin (shown as a black spot) to Lewy bodies is probably a secondary process to deposition. On the right are some of the proposed cellular targets for α-synuclein mediated toxicity, which include (from top to bottom) ER-golgi transport, synaptic vesicles, mitochondria and lysosomes and other proteolytic machinery. In each of these cases, it is proposed that α-synuclein has detrimental effects, listed below each arrow, although at this time it is not clear if any of these are either necessary or sufficient for toxicity in neurons.
Protein-protein interactions
Alpha-synuclein has been shown to interact with *Dopamine transporter
The dopamine transporter (DAT, also sodium-dependent dopamine transporter) is a membrane-spanning protein coded for in humans by the ''SLC6A3'' gene (also known as ''DAT1''), that pumps the neurotransmitter dopamine out of the synaptic cleft ba ...
,
* Parkin (ligase)
Parkin is a 465-amino acid residue E3 ubiquitin ligase, a protein that in humans and mice is encoded by the ''PARK2'' gene. Parkin plays a critical role in ubiquitination – the process whereby molecules are covalently labelled with ubiquitin ...
,
* Phospholipase D1
Phospholipase D1 (PLD1) is an enzyme that in humans is encoded by the ''PLD1'' gene, though analogues are found in plants, fungi, prokaryotes, and even viruses.
History
The possibility of PLD1 was first mentioned in 1947 by authors Hanahan an ...
,
* SNCAIP,
* Tau protein
The tau proteins (abbreviated from tubulin associated unit) form a group of six highly soluble protein isoforms produced by alternative splicing from the gene ''MAPT'' (microtubule-associated protein tau). They have roles primarily in maintainin ...
.
* Beta amyloid
Amyloid beta (Aβ, Abeta or beta-amyloid) denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease. The peptides derive from the amyloid-beta precursor pro ...
See also
*Synuclein
Synucleins are a family of soluble proteins common to vertebrates, primarily expressed in neural tissue and in certain tumors.
The name is a blend of the words "synapse" and "nucleus", as it was first found in the synapses in the electromotor n ...
* Contursi Terme - the village in Italy where a mutation in the α-synuclein gene led to a family history of Parkinson's disease
* Anti-α-synuclein drug
References
Further reading
* *External links
* * * {{Nerve tissue protein Peripheral membrane proteins Lewy body dementia