|
Zincate
In chemistry the term zincate may refer to several substances containing the element zinc: * usually the anion ZnO22−, more properly called tetrahydroxozincate or salts thereof, such as sodium zincate . * the polymeric anion n(OH)3−and its salts, for example NaZn(OH)3· H2O. * an oxide containing zinc and a less electronegative element e.g. Na2ZnO2. In the health supplement industry zincate may also mean a commercially available zinc supplement, typically formulated as zinc sulfate. Solutions prepared from dissolving zinc hydroxide or zinc oxide in a strong alkali like sodium hydroxide, which contains various zincate anions, are used in the metal plating industry, in processes such as immersion zinc plating and electroplating ( electrogalvanization). Any of these techniques may be called zincate process. Inorganic compound nomenclature In the naming of inorganic compounds, "-zincate" is a suffix that indicates that a polyatomic anion contains a central zinc atom. E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroxozincate
In chemistry, tetrahydroxozincate or tetrahydroxidozincate is a divalent anion (negative ion) with formula , with a central zinc atom in the +2 or (II) valence state coordinated to four hydroxide groups. It has Sp3 hybridization. It is the most common of the zincate anions, and is often called just zincate. These names are also used for the salts containing that anion, such as sodium zincate Na2Zn(OH)4 and calcium zincate CaZn(OH)4·2H2O Zincate salts can be obtained by reaction of zinc oxide (ZnO) or zinc hydroxide () and a strong base like sodium hydroxide. It is now generally accepted that the resulting solutions contain the tetrahydroxozincate ion. Earlier Raman studies had been interpreted as indicating the existence of linear ions. Related anions and salts The name "zincate" may also refer to a polymeric anion with formula approaching []''n'', which forms salts such as ·, or to mixed oxides of zinc and less electronegativity, electronegative elements, such as . S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
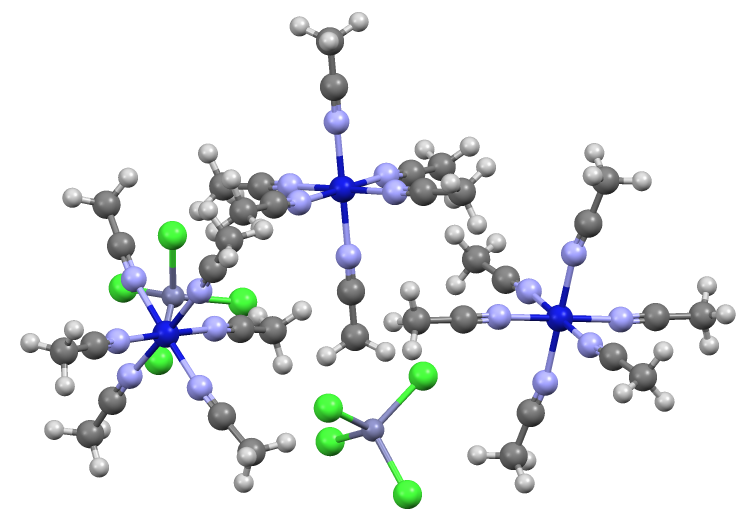
Tetrachlorozincate
Tetrachlorozincate is an anion with the formula nCl4sup>2−. It is a counterion that is often used in conjunction with strong electrophiles. Being dianionic, tetrachlorozincate is not classified as a weakly coordinating anion. On the other hand, being dianionic, tetrachlorozincate facilitates the crystallization of many salts. It has a tetrahedral molecular geometry. A simple example is (NH4)2 nCl4 The anion is tetrahedral. Zincates are anionic zinc complexes. Related to the preparation of Lucas' reagent, tetrachlorozincates are often generated by combining hydrochloric acid Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ... and zinc chloride. A related anion is n2Cl6sup>2−, in which again Zn(II) adopts a tetrahedral geometry. References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Zincate
Sodium zincate refers to anionic zinc oxides or hydroxides, depending on conditions. In the applications of these materials, the exact formula is not necessarily important and it is likely that aqueous zincate solutions consist of mixtures. Hydroxyzincates Solutions of sodium zincate may be prepared by dissolving zinc, zinc hydroxide, or zinc oxide in an aqueous solution of sodium hydroxide Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali .... Simplified equations for these complex processes are: :ZnO + H2O + 2 NaOH → Na2Zn(OH)4 :Zn + 2 H2O + 2 NaOH → Na2Zn(OH)4 + H2 From such solutions, one can crystallize salts of containing the anions Zn(OH)42−, Zn2(OH)62−, and Zn(OH)64−. Na2Zn(OH)4 consists of tetrahedral zincate ion and octahedral sodium cations. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immersion Zinc Plating
Immersion zinc plating is an electroless (non- electrolytic) coating process that deposits a thin layer of zinc on a less electronegative metal, by immersion in a solution containing a zinc or zincate ions, . A typical use is plating aluminum with zinc prior to electrolytic or electroless nickel plating. Immersion zinc plating involves the displacement of zinc from zincate by the underlying metal: :3 + 2 Al → 3 Zn + 2 + 4 OH− See also * Electrogalvanization (electrolytic zinc coating) * Immersion gold plating * Immersion copper plating Immersion may refer to: The arts * "Immersion", a 2012 story by Aliette de Bodard * ''Immersion'', a French comic book series by Léo Quievreux * ''Immersion'' (album), the third album by Australian group Pendulum * ''Immersion'' (film), a 202 ... * Immersion silver plating References Glenn O. Mallory, Juan B. Hajdu (1990), Electroless Plating: Fundamentals and Applications, American Electroplaters and Surface Finishers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Compounds
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript behavior, they are generally colorless (unlike other elements with the oxidation number +2, which are usually white), do not readily engage in redox reactions, and generally adopt symmetrical structures. General characteristics In its compounds, Zn2+ ions have an electronic configuration r3d10. As such, Zn2+ tends to have a symmetrical coordination geometry in both its complexes and compounds. In both ZnO and ZnS, (zincblende) zinc is bound tetrahedrally bound to four ligands (oxide and sulfide, respectively). Many complexes, such as ZnCl42−, are tetrahedral. Tetrahedrally coordinated zinc is found in metallo-enzymes such as carbonic anhydrase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrogalvanization
Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. The process involves electroplating, running a current of electricity through a saline/zinc solution with a zinc anode and steel conductor. Such Zinc electroplating or Zinc alloy electroplating maintains a dominant position among other electroplating process options, based upon electroplated tonnage per annum. According to the International Zinc Association, more than 5 million tons are used yearly for both hot dip galvanizing and electroplating. The plating of zinc was developed at the beginning of the 20th century. At that time, the electrolyte was cyanide based. A significant innovation occurred in the 1960s, with the introduction of the first acid chloride based electrolyte. The 1980s saw a return to alkaline electrolytes, only this time, without the use of cyanide. The most commonly used electrogalvanized cold rolled steel is SECC, acronym of "Steel, Electrogalvaniz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ate Complex
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom (from the Lewis acid) increases its valence and gains a negative formal charge. (In this definition, the meaning of valence is equivalent to coordination number). Often in chemical nomenclature the term ''ate'' is suffixed to the element in question. For example, the ate complex of a boron compound is called a borate. Thus trimethylborane and methyllithium react to form the ate compound , lithium tetramethylborate(1-). This concept was introduced by Georg Wittig in 1958. Ate complexes are common for metals, including the transition metals (groups 3-11), as well as the metallic or semi-metallic elements of group 2, 12, and 13. They are also well-established for third-period or heavier elements of groups 14–18 in their higher oxidation states. Ate complexes are a counterpart to onium ions. Lewis acids form ate ions when the central atom reacts with a donor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Hydroxide
Zinc hydroxide Zn( OH)2 is an inorganic chemical compound. It also occurs naturally as 3 rare minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal). Like the hydroxides of other metals, such as lead, aluminium, beryllium, tin and chromium, Zinc hydroxide (and Zinc oxide), is amphoteric. Thus it will dissolve readily in a dilute solution of a strong acid, such as HCl, and also in a solution of an alkali such as sodium hydroxide. Preparation It can be prepared by adding sodium hydroxide solution, but not in excess, to a solution of any zinc salt. A white precipitate will be seen: :Zn2+ + 2 OH− → Zn(OH)2. Zn2+ is known to form hexa-aqua ions at high water concentrations and tetra-aqua ions at low concentrations of water Sze, Yu-Keung, and Donald E. Irish. "Vibrational spectral studies of ion-ion and ion-solvent interactions. I. Zinc nitrate in water." Journal of Solution Chemistry 7.6 (1978): 395-415. and, thus, this reaction may be better ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the Chemical formula, formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, Zinc metabolism, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. ZnO is a wide-band gap semiconductor of the II-VI semiconductor, II-VI semiconductor group. The native doping (semiconductor), doping of the semiconductor due to oxygen vacancies or zinc interstitials is n-type. Other favorable properties include good transparency, high electron mobility, wide band gap, and strong room-temperature luminescence. Those properties make ZnO valuable for a variety of emerging applications: transparent electrodes in liquid crystal displays, energy-saving o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeitschrift Für Anorganische Und Allgemeine Chemie
The ''Zeitschrift für anorganische und allgemeine Chemie'' (''Journal of Inorganic and General Chemistry'') is a semimonthly peer-reviewed scientific journal covering inorganic chemistry, published by Wiley-VCH. The editors-in-chief are Thomas F. Fässler, Christian Limberg, Guodong Qian, and David Scheschkewitz. Originally the journal was published in German, but nowadays it is completely in English. Abstracting and indexing The journal is abstracted and indexed in the following databases: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 1.414, ranking it 40th out of 46 journals in the category "Chemistry, Inorganic & Nuclear". References External links * Chemistry journals Wiley-VCH aca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |