Tetrachlorozincate on:
[Wikipedia]
[Google]
[Amazon]
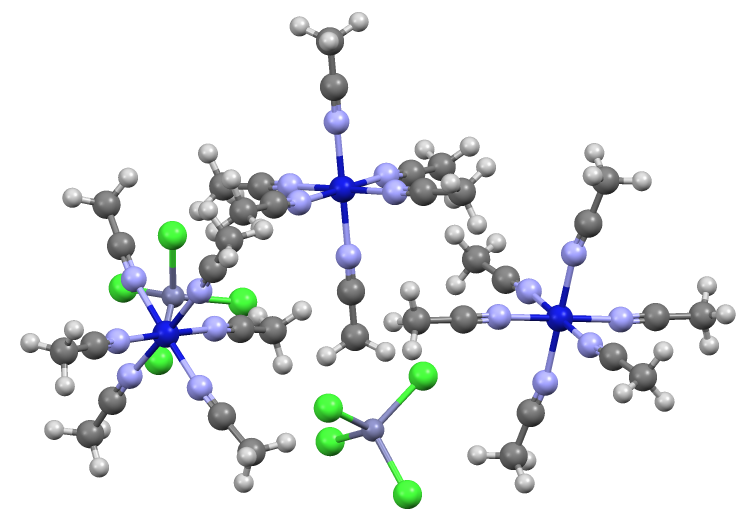 Tetrachlorozincate is an
Tetrachlorozincate is an  A related anion is n2Cl6sup>2−, in which again Zn(II) adopts a tetrahedral geometry.
A related anion is n2Cl6sup>2−, in which again Zn(II) adopts a tetrahedral geometry.
 Tetrachlorozincate is an
Tetrachlorozincate is an anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
with the formula nCl4sup>2−. It is a counterion that is often used in conjunction with strong electrophiles. Being dianionic, tetrachlorozincate is not classified as a weakly coordinating anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found ...
. On the other hand, being dianionic, tetrachlorozincate facilitates the crystallization of many salts. It has a tetrahedral molecular geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccosine, cos−1(−) = 109.4712206...° ≈ 109.5° when all four substit ...
. A simple example is (NH4)2 nCl4 The anion is tetrahedral. Zincates are anionic zinc complexes.
Related to the preparation of Lucas' reagent, tetrachlorozincates are often generated by combining hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
and zinc chloride.
 A related anion is n2Cl6sup>2−, in which again Zn(II) adopts a tetrahedral geometry.
A related anion is n2Cl6sup>2−, in which again Zn(II) adopts a tetrahedral geometry.
References