|
Van Der Waals Strain
Van der Waals strain is strain resulting from Van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their Van der Waals radii. Van der Waals strain is also called Van der Waals repulsion and is related to steric hindrance. One of the most common forms of this strain is eclipsing hydrogen, in alkanes. In rotational and pseudorotational mechanisms In molecules whose vibrational mode involves a rotational or pseudorotational mechanism (such as the Berry mechanism or the Bartell mechanism), Van der Waals strain can cause significant differences in potential energy, even between molecules with identical geometry. PF5, for example, has significantly lower potential energy than PCl5. Despite their identical trigonal bipyramidal molecular geometry, the higher electron count of chlorine as compared to fluorine causes a potential energy spike as the molecule enters its intermediate in the mechanism and the substituen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compressed spring.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released. Summary Thermodynamics The equilibrium of two molecular conformations is determined by the difference in Gibbs free energy of the two conformations. From this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potential Energy
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors. Common types of potential energy include the gravitational potential energy of an object, the elastic potential energy of an extended spring, and the electric potential energy of an electric charge in an electric field. The unit for energy in the International System of Units (SI) is the joule, which has the symbol J. The term ''potential energy'' was introduced by the 19th-century Scottish engineer and physicist William Rankine, although it has links to Greek philosopher Aristotle's concept of potentiality. Potential energy is associated with forces that act on a body in a way that the total work done by these forces on the body depends only on the initial and final positions of the body in space. These forces, that are called ''conservative forces'', can be represented at every point in space by vec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Surface
The Van der Waals surface of a molecule is an abstract representation or model of that molecule, illustrating where, in very rough terms, a surface might reside for the molecule based on the hard cutoffs of Van der Waals radii for individual atoms, and it represents a surface through which the molecule might be conceived as interacting with other molecules. Also referred to as a ''Van der Waals envelope,'' the Van der Waals surface is named for Johannes Diderik van der Waals, a Dutch theoretical physicist and thermodynamicist who developed theory to provide a liquid-gas equation of state that accounted for the non-zero volume of atoms and molecules, and on their exhibiting an attractive force when they interacted (theoretical constructions that also bear his name). Van der Waals surfaces are therefore a tool used in the abstract representations of molecules, whether accessed, as they were originally, via hand calculation, or via physical wood/plastic models, or now digitally, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Molecule
A Van der Waals molecule is a weakly bound complex of atoms or molecules held together by intermolecular attractions such as Van der Waals forces or by hydrogen bonds. The name originated in the beginning of the 1970s when stable molecular clusters were regularly observed in molecular beam microwave spectroscopy. Examples Examples of well-studied vdW molecules are Ar2, H2-Ar, H2O-Ar, benzene-Ar, (H2O)2, and (HF)2. Others include the largest diatomic molecule: He2 and LiHe. Supersonic beam spectroscopy In (supersonic) molecular beams temperatures are very low (usually less than 5 K). At these low temperatures Van der Waals (vdW) molecules are stable and can be investigated by microwave, far-infrared spectroscopy and other modes of spectroscopy. Also in cold equilibrium gases vdW molecules are formed, albeit in small, temperature dependent concentrations. Rotational and vibrational transitions in vdW molecules have been observed in gases, mainly by UV and IR spectro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der Waals contact distance; this phenomenon resul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
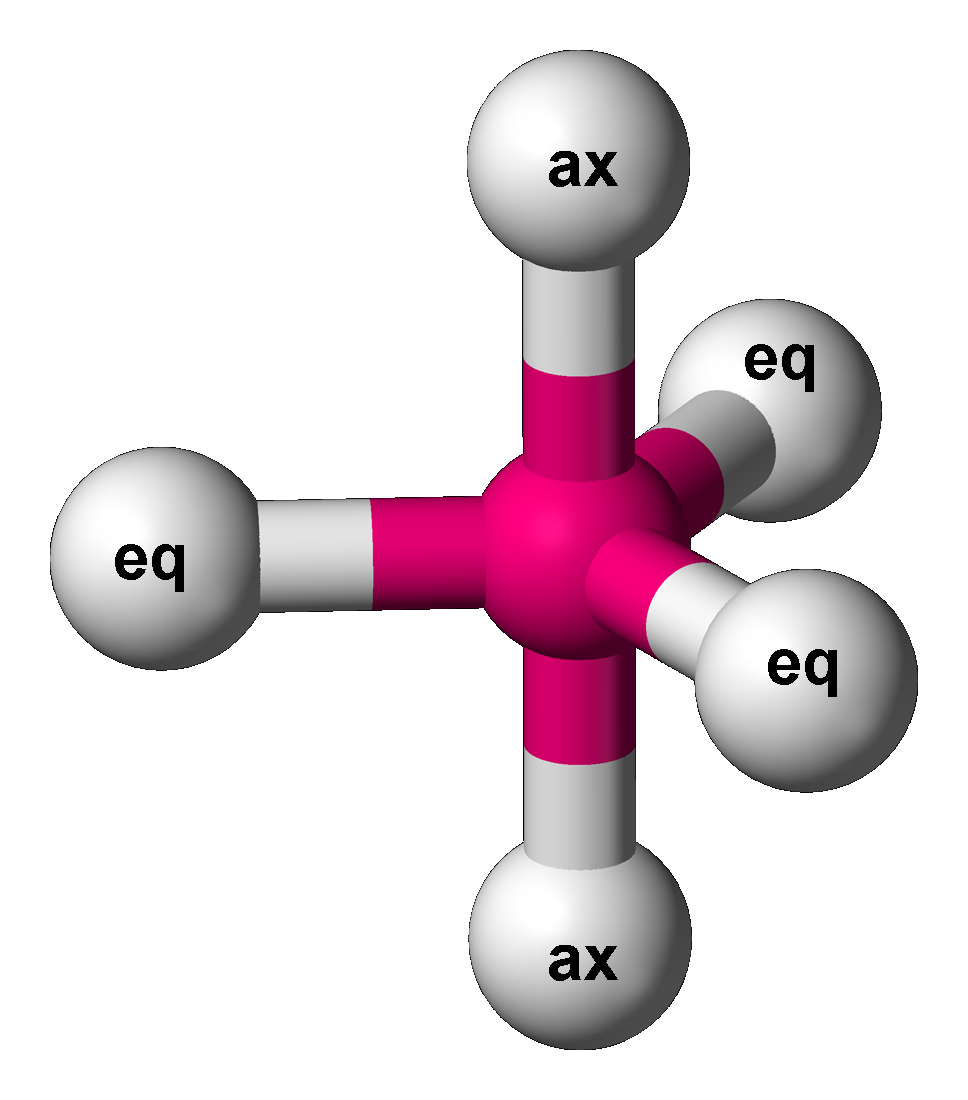
Trigonal Bipyramidal Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crowd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCl5
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride. Structure The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of PCl5 depends on its environment. Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (''D''3h) symmetry. The hypervalent nature of this species (as well as of , see below) can be explained with the inclusion of non-bonding molecular orbitals (molecular orbital theory) or resonance (valence bond theory). This trigonal bipyramidal structure persists in nonpolar solvents, such as CS2 and CCl4. In the solid state PCl5 is an ionic compound, formulated . In solutions of polar solvents, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bartell Mechanism
The Bartell mechanism is a pseudorotational mechanism similar to the Berry mechanism. It occurs only in molecules with a pentagonal bipyramidal molecular geometry, such as IF7. This mechanism was first predicted by H. B. Bartell. The mechanism exchanges the axial atoms with one pair of the equatorial atoms with an energy requirement of about 2.7 kcal/mol. Similarly to the Berry mechanism in square planar molecules, the symmetry of the intermediary phase of the vibrational mode is "chimeric"LS Bartell, MJ Rothman & A Gavezzotti, 1982, , ''J. Chem. Phys.'' 76:4136-4413.M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Educ.'' (online), 2005; se, accessed 28 May 2014 of other mechanisms; it displays characteristics of the Berry mechanism, a "lever" mechanism seen in pseudor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der Waals contact distance; this phenomenon resul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berry Mechanism
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.RS Berry, 1960, "Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds," ''J. Chem. Phys.'' 32:933-938, DOI 10.1063/1.1730820; seo accessed 28 May 2014M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Educ. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |