|
Voxilaprevir
Voxilaprevir is a hepatitis C virus (HCV) nonstructural (NS) protein 3/ 4A protease inhibitor (by Gilead) that is used in combination with sofosbuvir and velpatasvir. The combination has the trade name Vosevi and received a positive opinion from the European Committee for Medicinal Products for Human Use in June 2017. On 18 July 2017, Vosevi was approved by the US Food and Drug Administration The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respon .... References NS3/4A protease inhibitors Tert-butyl compounds {{antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vosevi
Sofosbuvir/velpatasvir/voxilaprevir, sold under the brand name Vosevi, is a fixed-dose combination medication for the treatment of hepatitis C. It combines three drugs that each act by a different mechanism of action against the hepatitis C virus: sofosbuvir, velpatasvir, and voxilaprevir.Vosevi was approved for medical use in the United States and in the European Union in July 2017. Vosevi is sold by Gilead Sciences Gilead Sciences, Inc. () is an American biopharmaceutical company headquartered in Foster City, California, that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and CO .... References External links * Combination drugs NS3/4A protease inhibitors NS5B (polymerase) inhibitors NS5A inhibitors {{antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Velpatasvir
Velpatasvir is an NS5A inhibitor (by Gilead) which is used together with sofosbuvir in the treatment of hepatitis C infection of all six major genotypes. Side effects Side effects in studies occurred with similar frequencies as in people treated with placebo. Interactions Velpatasvir is both an inhibitor and a substrate of the transporter proteins P-glycoprotein (Pgp), ABCG2, OATP1B1 and OATP1B3. It is partly degraded by the liver enzymes CYP2B6, CYP2C8 and CYP3A4. Substances that are transported or inactivated by these proteins, or interfere with them, can interact with velpatasvir. In studies, this has been found for the HIV combination efavirenz/emtricitabine/tenofovir, which reduces the area under the curve (AUC) of velpatasvir by about 50%, and the CYP3A4 and Pgp inducer rifampicin, which reduces its AUC by about 80%, rendering it likely ineffective. Digoxin is eliminated by Pgp; its AUC is increased by about 30% in combination with velpatasvir and sofosbuvir (although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilead Sciences
Gilead Sciences, Inc. () is an American biopharmaceutical company headquartered in Foster City, California, that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500. Gilead was founded in 1987 under the name Oligogen by Michael L. Riordan. The original name was a reference to oligomers, small strands of DNA used to target genetic sequences. Gilead held its IPO in 1992, and successfully developed drugs like Tamiflu and Vistide that decade. In the 2000s, Gilead received approval for drugs including Viread and Hepsera, among others. It began evolving from a biotechnology company into a pharmaceutical company, acquiring several subsidiaries, though it still relied heavily on contracting to manufacture its drugs. The company continued its growth in the 2010s, but came under heavy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sofosbuvir
Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken Oral administration, by mouth. Common side effects include fatigue, headache, nausea, and trouble sleeping. Side effects are generally more common in interferon-containing regimens. Sofosbuvir may reactivate hepatitis B in those who have been previously infected. In combination with ledipasvir, daclatasvir or simeprevir, it is not recommended with amiodarone due to the risk of an bradycardia, abnormally slow heartbeat. Sofosbuvir is in the nucleotide analog family of medications and works by blocking the hepatitis C NS5B protein. Sofosbuvir was discovered in 2007 and approved for medical use in the United States in 2013. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Medical uses Initial HCV treatment In 2016, the American Association for the Study of Liver Diseases and the Infectious Diseases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
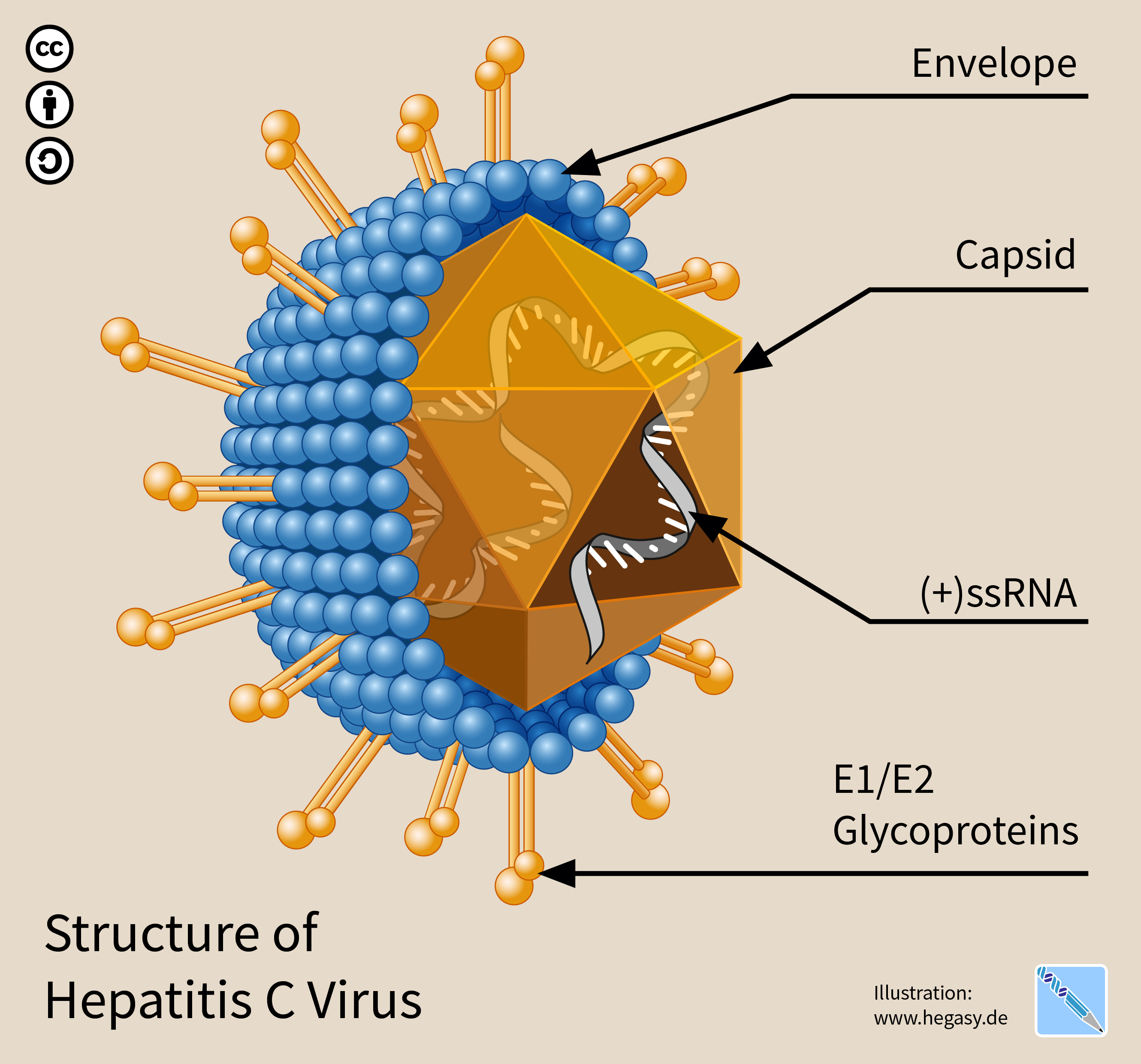
Hepatitis C Virus
The hepatitis C virus (HCV) is a small (55–65 nm in size), enveloped, positive-sense single-stranded RNA virus of the family ''Flaviviridae''. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer ( hepatocellular carcinoma, abbreviated HCC) and lymphomas in humans. Taxonomy The hepatitis C virus belongs to the genus ''Hepacivirus'', a member of the family ''Flaviviridae''. Before 2011, it was considered to be the only member of this genus. However a member of this genus has been discovered in dogs: canine hepacivirus. There is also at least one virus in this genus that infects horses. Several additional viruses in the genus have been described in bats and rodents. Structure The hepatitis C virus particle consists of a lipid membrane envelope that is 55 to 65 nm in diameter. Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope. They take part in viral attachment and entry into the cell. Within the envel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NS3 (HCV)
Nonstructural protein 3 (NS3), also known as p-70, is a viral nonstructural protein that is 70 kDa cleavage product of the hepatitis C virus polyprotein. It acts as a serine protease. C-terminal two-thirds of the protein also acts as helicase and nucleoside triphosphatase. First (N-terminal) 180 aminoacids of NS3 has additional role as cofactor domains for NS2 protein. See also * Boceprevir, sovaprevir, paritaprevir and telaprevir Telaprevir (VX-950), marketed under the brand names Incivek and Incivo, is a pharmaceutical drug for the treatment of hepatitis C co-developed by Vertex Pharmaceuticals and Johnson & Johnson. It is a member of a class of antiviral drugs known as ... - drugs targeting this protein References External links * http://www.uniprot.org/uniprot/Q91RS4 Viral nonstructural proteins Hepatitis C virus {{virus-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NS4A
Nonstructural protein 4A (NS4A) is a viral protein found in the hepatitis C virus The hepatitis C virus (HCV) is a small (55–65 nm in size), enveloped, positive-sense single-stranded RNA virus of the family ''Flaviviridae''. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer ( hepato .... It acts as a cofactor for the enzyme NS3. References Viral nonstructural proteins Hepatitis C virus {{virus-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)