|
Trimethylindium
Trimethylindium, often abbreviated to TMI or TMIn, is the organoindium compound with the formula In(CH3)3. It is a colorless, pyrophoric solid. Unlike trimethylaluminium, but akin to trimethylgallium, TMI is monomeric. Preparation TMI is prepared by the reaction of indium trichloride with methyl lithium. : InCl3 + 3LiMe → Me3In.OEt2 + 3LiCl Properties Compared to trimethylaluminium and trimethylgallium, InMe3 is a weaker Lewis acid. It forms adducts with secondary amines and phosphines. A complex with the heterocyclic triazine ligand (PriNCH2)3 forms a complex with 6-coordinate In, where the C-In-C angles are 114°-117° with three long bonds to the tridentate ligand with N-In-N angles of 48.6° and long In-N bonds of 278 pm. Structure In the gaseous state InMe3 is monomeric, with a trigonal planar structure, and in benzene solution it is tetrameric.''CVD of compound semiconductors, Precursor Synthesis, Development and Applications'', Anthony C. Jones, Paul O'Brien, John ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylindium
Triethylindium is an organometallic compound. Its chemical formula is . Synthesis This compound can be obtained by reacting indium(III) chloride with a diethyl ether solution of ethyl magnesium chloride: : + 3 → In(C2H5)3 + 3 Other routes are also known. Properties Indium triethyl is a colorless, toxic, oxidation and hydrolysis-sensitive liquid. It is a monomer in the gaseous and dissolved state. The compound reacts with halomethanes to form diethyl indium halides. Triethylindium is highly reactive with water: :In(C2H5)3 + → In(C2H5)2OH + ↑ Applications Indium triethyl is used to prepare indium phosphide layers for microelectronics Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre-s .... See also * Trimethylindium References {{Indium compounds Indium compounds O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylgallium
Trimethylgallium, often abbreviated to TMG or TMGa, is the organogallium compound with the formula Ga(CH3)3. It is a colorless, pyrophoric liquid. Unlike trimethylaluminium, TMG adopts a monomeric structure. When examined in detail, the monomeric units are clearly linked by multiple weak Ga---C interactions, reminiscent of the situation for trimethylindium. Preparation Two forms of TMG are typically investigated: Lewis base adducts or TMG itself. All are prepared by reactions of gallium trichloride with various methylating agents. When the methylation is conducted with methylmagnesium iodide in diethyl ether, the product is the poorly volatile diethyl ether adduct is produced. The ether ligand is not readily lost, although it may be displaced with liquid ammonia. When the alkylation is conducted with methyl lithium in the presence of a tertiary phosphine the air-stable phosphine adduct is obtained: : Heating the solid phosphine adduct under vacuum liberates the base-free ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Trichloride
Indium(III) chloride is the chemical compound with the formula In Cl3. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. Synthesis and structure Being a relatively electropositive metal, indium reacts quickly with chlorine to give the trichloride. Indium trichloride is very soluble and deliquescent. A synthesis has been reported using an electrochemical cell in a mixed methanol-benzene solution. Like AlCl3 and TlCl3, InCl3 crystallizes as a layered structure consisting of a close-packed chloride arrangement containing layers of octahedrally coordinated In(III) centers,Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier a structure akin to that seen in YCl3.Wells, A.F. Structural Inorganic Chemistry, Oxford: Clarendon Press, 1984. . In contrast, GaCl3 crystallizes as dimers containing Ga2Cl6. Molten InCl3 conducts electricity, whereas AlCl3 do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MOVPE
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures (10 to 760 Torr). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as Light-emitting diodes. It was invented in 1968 at North American Aviation (later Rockwell International) Science Center by Harold M. Manasevit. Basic principles In MOCVD ultrapure precursor gases are injected into a reacto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalorganic Vapour Phase Epitaxy
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures (10 to 760 Torr). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as Light-emitting diodes. It was invented in 1968 at North American Aviation (later Rockwell International) Science Center by Harold M. Manasevit. Basic principles In MOCVD ultrapure precursor gases are injected into a re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compound Semiconductor
Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of their application in the computer and photovoltaic industry—in devices such as transistors, lasers, and solar cells—the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. Most commonly used semiconductor materials are crystalline inorganic solids. These materials are classified according to the periodic table groups of their constituent atoms. Different semiconductor materials differ in their properties. Thus, in comparison with silicon, compound semiconductors have both advantages and disadvantages. For example, gallium arsenide (GaAs) has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allows o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapour Deposition Precursors
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances (substances consisting of a single chemical element), chemical compounds, or alloys. Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a chemical substance is pure water; it has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory. Other chemical substances commonly encountered in pure form are diamond (carbon), gold, table salt (sodium chloride) and refined sugar (sucrose). However, in practice, no substance is entirely pure, and chemical purity is specified according to the intended use of the chemical. Chemical substances exist as solid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 greatest scientists of all time, and as of 2000, he was rated the 16th most important scientist in history. For his scientific work, Pauling was awarded the Nobel Prize in Chemistry in 1954. For his peace activism, he was awarded the Nobel Peace Prize in 1962. He is one of five people to have won more than one Nobel Prize (the others being Marie Curie, John Bardeen, Frederick Sanger and Karl Barry Sharpless). Of these, he is the only person to have been awarded two unshared Nobel Prizes, and one of two people to be awarded Nobel Prizes in different fields, the other being Marie Curie. Pauling was one of the founders of the fields of quantum chemistry and molecular biology. His contributions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure. The vapor pressure of any substance increases non-linearly with temperature accord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties. Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year. Indium is a minor component in zinc sulfide ores and is produced as a byproduct of zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conduct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Gallium Indium Phosphide
Aluminium gallium indium phosphide (, also AlInGaP, InGaAlP, GaInP, etc.) is a semiconductor material that provides a platform for the development of novel multi-junction photovoltaics and optoelectronic devices, as it spans a direct bandgap from deep ultraviolet to infrared. AlGaInP is used in manufacture of light-emitting diodes of high-brightness red, orange, green, and yellow color, to form the heterostructure emitting light. It is also used to make diode lasers. Formation AlGaInP layer is often grown by heteroepitaxy on gallium arsenide or gallium phosphide in order to form a quantum well structure. Heteroepitaxy is a kind of epitaxy performed with materials that are different from each other. In heteroepitaxy, a crystalline film grows on a crystalline substrate or film of a different material. This technology is often used to grow crystalline films of materials for which single crystals cannot 1D view. Another example of heteroepitaxy is gallium nitride (GaN) on sap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
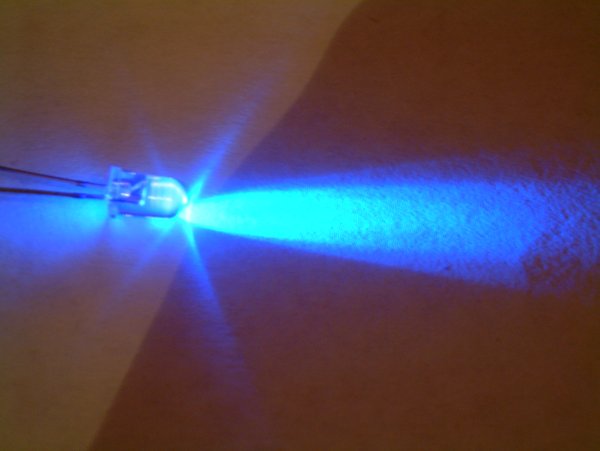
InGaN
Indium gallium nitride (InGaN, ) is a semiconductor material made of a mix of gallium nitride (GaN) and indium nitride (InN). It is a ternary group III/ group V direct bandgap semiconductor. Its bandgap can be tuned by varying the amount of indium in the alloy. InxGa1−xN has a direct bandgap span from the infrared (0.69 eV) for InN to the ultraviolet (3.4 eV) of GaN. The ratio of In/Ga is usually between 0.02/0.98 and 0.3/0.7. Applications LEDs Indium gallium nitride is the light-emitting layer in modern blue and green LEDs and often grown on a GaN buffer on a transparent substrate as, e.g. sapphire or silicon carbide. It has a high heat capacity and its sensitivity to ionizing radiation is low (like other group III nitrides), making it also a potentially suitable material for solar photovoltaic devices, specifically for arrays for satellites. It is theoretically predicted that spinodal decomposition of indium nitride should occur for compositions between 15% and 85%, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |