|
Triazoles
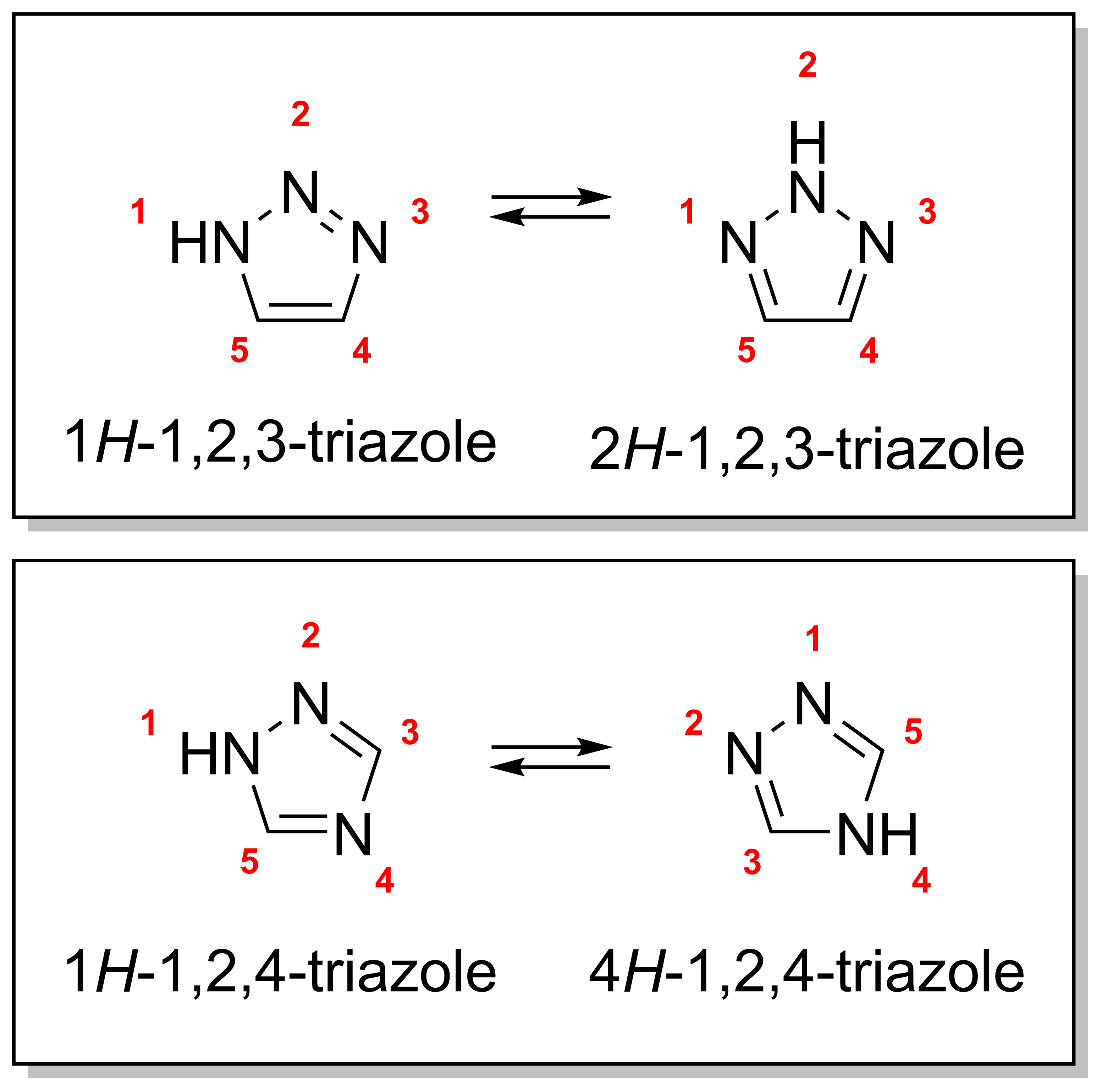
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring. Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as haptic ligands. Isomerism There are four triazole isomers, which are conventionally divided into two pairs of tautomers. In the 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-triazoles, an interstitial carbon separates out one nitrogen atom. Each category has two tautomers that differ by which nitrogen has a hydrogen bonded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2,4-Triazole
1,2,4-Triazole (as ligand in coordination compounds, Htrz abbreviation is sometimes used) is one of a pair of isomeric chemical compounds with molecular formula CHN, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,4-Triazole and its derivatives find use in a wide variety of applications. Structure and properties 1,2,4-Triazole is a planar molecule. The C-N and N-N distances fall into a narrow range 136 - 132 picometers, consistent with the aromaticity. Although two tautomers can be envisioned, only one exists practically speaking. 1,2,4-Triazole is amphoteric, being susceptible to both N-protonation and deprotonation in aqueous solution. The pKa of 1,2,4-triazolium (C2N3H4+) is 2.45. The pKa of the neutral molecule is 10.26. Synthesis and occurrence 1,2,4-Triazoles can be prepared using the Einhorn–Brunner reaction or the Pellizzari reaction. Unsubstituted 1,2,4-triazole can be prepared from thiosemicarbazide by acylation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Click Chemistry
In chemical synthesis, click chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules. Click reactions occur in one pot, are not disturbed by water, generate minimal and inoffensive byproducts, and are "spring-loaded"—characterized by a high thermodynamic driving force that drives it quickly and irreversibly to high yi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pellizzari Reaction
The Pellizzari reaction was discovered in 1911 by Guido Pellizzari, and is the organic reaction of an amide and a hydrazide to form a 1,2,4-triazole. The product is similar to that of the Einhorn-Brunner reaction, but the mechanism itself is not regioselective. : Mechanism The mechanism begins by the nitrogen in the hydrazide attacking the carbonyl carbon on the amide to form compound 3. The negatively charged oxygen then abstracts two hydrogens from neighboring nitrogens in order for a molecule of water to be released to form compound 5. The nitrogen then performs an intramoleculer attack on the carbonyl group to form the five-membered ring of compound 6. After another proton migration from the nitrogens to the oxygen, another water molecule is released to form the 1,2,4-triazole 8. : Uses The synthesis of the 1,2,4-triazole has a wide range of biological functions. 1,2,4-triazoles have antibacterial, antifungal, antidepressant and hypoglycemic Hypoglycemia, also called low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Huisgen 1,3-dipolar Cycloaddition
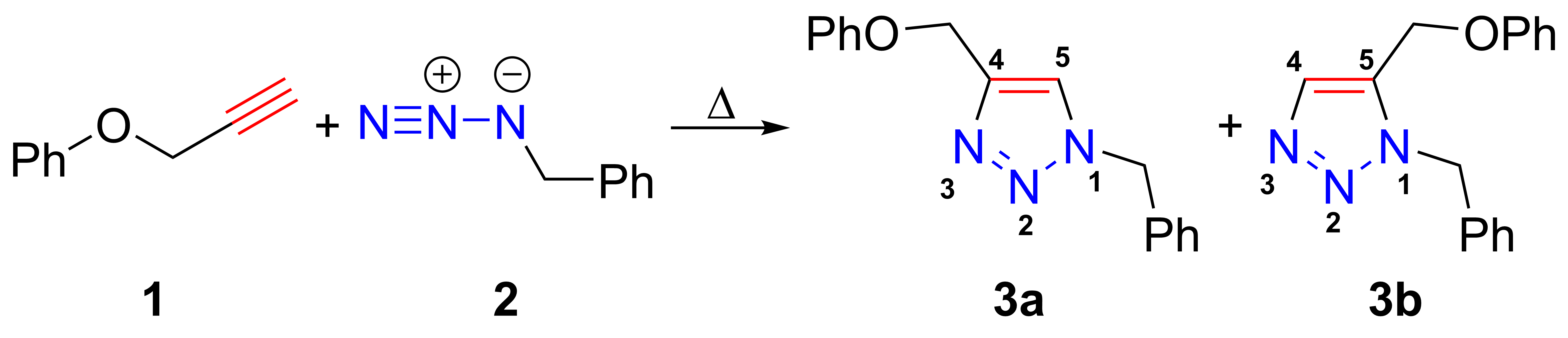
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction". In the reaction above azide 2 reacts neatly with alkyne 1 to afford the product triazole as a mixture of 1,4-adduct (3a) and 1,5-adduct (3b) at 98 °C in 18 hours. The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene as dipolarophile has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes. Although azides are not the most reactive 1,3-dipole available for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifungal Drug
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually yes obtained by a doctor's prescription, but a few are available over the counter (OTC). Types of antifungal There are two types of antifungals: local and systemic. Local antifungals are usually administered topically or vaginally, depending on the condition being treated. Systemic antifungals are administered orally or intravenously. Of the clinically employed azole antifungals, only a handful are used systemically. These include ketoconazole, itraconazole, fluconazole, fosfluconazole, voriconazole, posaconazole, and isavuconazole. Examples of non-azole systemic antifungals include griseofulvin and terbinafine. Classes Polyenes A polyene is a molecule with multiple conjugated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Satan
Satan,, ; grc, ὁ σατανᾶς or , ; ar, شيطانالخَنَّاس , also known as Devil in Christianity, the Devil, and sometimes also called Lucifer in Christianity, is an non-physical entity, entity in the Abrahamic religions that seduces humans into sin or falsehood. In Judaism, Satan is seen as an agent subservient to God in Judaism, God, typically regarded as a metaphor for the ''yetzer hara'', or "evil inclination." In Christianity and Islam, he is usually seen as a fallen angel or jinn who has rebelled against God in Abrahamic religions, God, who nevertheless allows him temporary power over the fallen world and a host of demons. In the Quran, Shaitan, also known as Iblis, is an entity made of fire who was cast out of Heaven because he refused to bow before the newly created Adam in Islam, Adam and incites humans to sin by infecting their minds with ''waswās'' ("evil suggestions"). A figure known as ''ha-satan'' ("the satan") first appears in the Hebrew B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazines
Hydrazines (R2N−NR2) are a class of chemical compounds with two nitrogen atoms linked via a covalent bond and which carry from one up to four alkyl or aryl substituents. Hydrazines can be considered as derivatives of the inorganic hydrazine (H2N−NH2), in which one or more hydrogen atoms have been replaced by hydrocarbon groups. Production * 1,1-Dimethylhydrazine is produced by the reduction of ''N''-nitrosodimethylamine. Siegfried Hauptmann: ''Organische Chemie'', 2. durchgesehene Auflage, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, S. 522–523, . * The reduction of benzenediazonium chloride with tin(II) chloride and hydrochloric acid provides phenylhydrazine. * 2,4-Dinitrophenylhydrazine is produced by the reaction of 1-chloro-2,4-dinitrobenzene with hydrazine. * Tetraphenylhydrazine is formed by the oxidation of diphenylamine with potassium permanganate in acetone. Classification Hydrazines can be divided into three groups according to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Chemistry
Green chemistry, also called sustainable chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental chemistry focuses on the effects of polluting chemicals on nature, green chemistry focuses on the environmental impact of chemistry, including lowering consumption of nonrenewable resources and technological approaches for preventing pollution. The overarching goals of green chemistry—namely, more resource-efficient and inherently safer design of molecules, materials, products, and processes—can be pursued in a wide range of contexts. History Green chemistry emerged from a variety of existing ideas and research efforts (such as atom economy and catalysis) in the period leading up to the 1990s, in the context of increasing attention to problems of chemical pollution and resource depletion. The development of green chemistry in Europe a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" * Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel *CHEM-DT CHEM-DT is the TVA owned-and-operated television station in Trois-Rivières, Quebec, Canada. It broadcasts a high-definition digital signal on VHF channel 8 from a transmitter on Rue Principale in Notre-Dame-du-Mont-Carmel. Owned by the Grou ..., a Canadian television channel See also * Chemo (other) * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzotriazole
Benzotriazole (BTA) is a heterocyclic compound with the chemical formula C6H5N3. Its five-membered ring contains three consecutive nitrogen atoms. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and triazole. This white-to-light tan solid has a variety of uses, for instance, as a corrosion inhibitor for copper. Structure Benzotriazole features two fused rings. Its five-membered ring can exist in tautomers A and B, and the derivatives of both tautomers, structures C and D, can also be produced: : Various structural analyses with UV, IR and 1H-NMR spectra indicated that isomer A is predominantly present at room temperature. The bond between positions 1 and 2 and the one between positions 2 and 3 have proved to have the same bond properties. Moreover, the proton does not tightly bind to any of the nitrogen atoms, but rather migrates rapidly between positions 1 and 3. Therefore, the BTA can lose a proton to act as a weak acid (pKa = 8.2) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3_(FIBCEA01).png)