|
Transformer Oil Testing
Transformer oil, a type of insulating and cooling oil used in transformers and other electrical equipment, needs to be tested periodically to ensure that it is still fit for purpose. This is because it tends to deteriorate over time. Testing sequences and procedures are defined by various international standards, many of them set by ASTM. Transformer oil testing consists of measuring breakdown voltage and other physical and chemical properties of samples of the oil, either in a laboratory or using portable test equipment on-site. Motivation for testing The transformer oil (insulation oil) of voltage transformers and current transformers fulfills the purpose of insulating as well as cooling. Thus, the dielectric quality of transformer oil is essential to secure operation of a transformer. As transformer oil deteriorates through aging and moisture ingress, transformer oil should, depending on economics, transformer duty and other factors, be tested periodically. Electric utility co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transformer Oil
Transformer oil or insulating oil is an oil that is stable at high temperatures and has excellent electrical insulating properties. It is used in oil-filled transformers (wet transformers), some types of high-voltage capacitors, fluorescent lamp ballasts, and some types of high-voltage switches and circuit breakers. Its functions are to insulate, suppress corona discharge and arcing, and to serve as a coolant. Transformer oil is most often based on mineral oil, but alternative formulations with different engineering or environmental properties are growing in popularity. Function and properties Transformer oil's primary functions are to insulate and cool a transformer. It must therefore have high dielectric strength, thermal conductivity, and chemical stability, and must keep these properties when held at high temperatures for extended periods. Typical specifications are: flash point 140 °C or greater, pour point −40 °C or lower, dielectric breakdown voltage 28 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
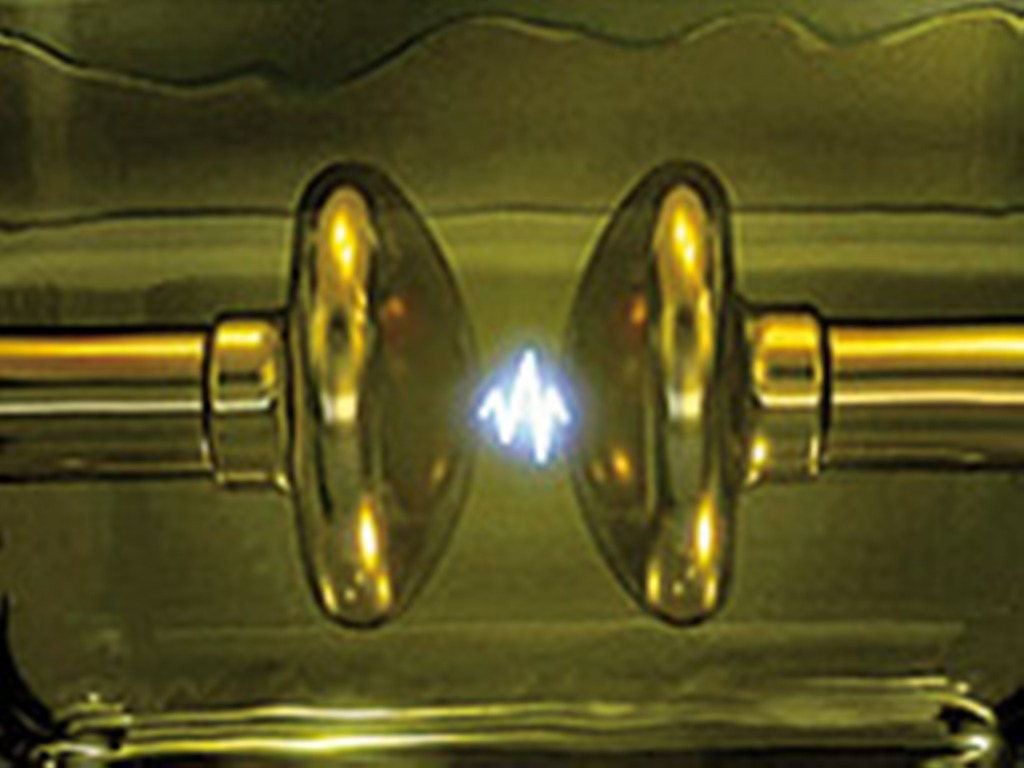
Flash Point
The flash point of a material is the "lowest liquid temperature at which, under certain standardized conditions, a liquid gives off vapours in a quantity such as to be capable of forming an ignitable vapour/air mixture". (EN 60079-10-1) The flash point is sometimes confused with the autoignition temperature, the temperature that causes spontaneous ignition. The fire point is the lowest temperature at which the vapors keep burning after the ignition source is removed. It is higher than the flash point, because at the flash point vapor may not be produced fast enough to sustain combustion. Neither flash point nor fire point depends directly on the ignition source temperature, but ignition source temperature is far higher than either the flash or fire point. Fuels The flash point is a descriptive characteristic that is used to distinguish between flammable fuels, such as petrol (also known as gasoline), and combustible fuels, such as diesel. It is also used to characterize the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Severity Factor
A severity factor is established as a coefficient to assess the dielectric severity supported by a transformer winding considering the incoming transient overvoltage (voltage spike). It determines the safety margin regarding to the standard acceptance tests either in the frequency or time domain. Severity factors are a newly concept for analyzing the dielectric severity supported along transformer windings when a transformer is submitted to a non-standardized transient voltage waveform induced from the power system. Two are the new factors considered for evaluating the severity supported by the insulation windings both in factory and in service. One factor is called Time Domain Severity Factor (TDSF) and another one is the Frequency Domain Severity Factor (FDSF). Background One first approach to the concept of severity factor was made by Malewski et al. Later, Asano et al. applied the Malewski's idea for further analysis but including the concept of Energy Spectral Density (ESD) as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Measurements
Electrical measurements are the methods, devices and calculations used to measure electrical quantities. Measurement of electrical quantities may be done to measure electrical parameters of a system. Using transducers, physical properties such as temperature, pressure, flow, force, and many others can be converted into electrical signals, which can then be conveniently measured and recorded. High-precision laboratory measurements of electrical quantities are used in experiments to determine fundamental physical properties such as the charge of the electron or the speed of light, and in the definition of the units for electrical measurements, with precision in some cases on the order of a few parts per million. Less precise measurements are required every day in industrial practice. Electrical measurements are a branch of the science of metrology. Measurable independent and semi-independent electrical quantities comprise: * Voltage * Electric current * Electrical resistance and el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek letter (rho). The SI unit of electrical resistivity is the ohm-meter (Ω⋅m). For example, if a solid cube of material has sheet contacts on two opposite faces, and the resistance between these contacts is , then the resistivity of the material is . Electrical conductivity or specific conductance is the reciprocal of electrical resistivity. It represents a material's ability to conduct electric current. It is commonly signified by the Greek letter ( sigma), but (kappa) (especially in electrical engineering) and (gamma) are sometimes used. The SI unit of electrical conductivity is siemens per metre (S/m). Resistivity and conductivity are intensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecation, deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relative Density
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance with a relative density greater t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polychlorinated Biphenyl
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1979 and internationally by the Stockholm Convention on Persistent Organic Pollutants in 2001. They are organic chlorine compounds with the formula C12 H10−''x'' Cl''x''; they were once widely used in the manufacture of carbonless copy paper, as heat transfer fluids, and as dielectric and coolant fluids for electrical equipment. Because of their longevity, PCBs are still widely in use, even though their manufacture has declined drastically since the 1960s, when a host of problems were identified. With the discovery of PCBs' environmental toxicity, and classification as persistent organic pollutants, their production was banned by United States federal law in 1978, and by the Stockholm Convention on Persistent Organic Pollutants in 2001. The International Agency ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation Inhibitor
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricants, to prevent oxidation, and to foods to prevent spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress. The only dietary antioxidants are vitamins A, C, and E, but the term ''antioxidant'' has also been applied to numerous other dietary compounds that only have antioxidant properties in vitro, with little evidence for antioxidant properties in vivo. Dietary supplements marketed as antioxidants have not been shown to maintain health or prevent disease in humans. History As part of their adaptation from marine life, terrestrial plants began producing non-marine antioxidants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutralization (chemistry)
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants. Meaning of "neutralization" In the context of a chemical reaction the term neutralization is used for a reaction between an acid and a base or alkali. Historically, this reaction was represented as :acid + base (alkali) → salt + water For example: :HCl + NaOH → NaCl + H2O The statement is still valid as long as it is understood that in an aqueous solution the substances involved are subject to dissociation, which changes the ionization state of the substances. The arrow sign, →, is used because the reaction is complete, that is, neutralization is a quantitative reaction. A more general definition is base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karl Fischer Titration
Karl Fischer titration is a classic titration method in chemical analysis that uses coulometric or volumetric titration to determine trace amounts of water in a sample. It was invented in 1935 by the German chemist Karl Fischer. Today, the titration is done with an automated Karl Fischer titrator. Chemical principle The elementary reaction responsible for water quantification in the Karl Fischer titration is oxidation of sulfur dioxide with iodine: : 2 H2O + SO2 + I2 → H2SO4 + 2 HI This elementary reaction consumes exactly one molar equivalent of water vs. iodine. Iodine is added to the solution until it is present in excess, marking the end point of the titration, which can be detected by potentiometry. The reaction is run in an alcohol solution containing a base, which consumes the sulfur trioxide and hydroiodic acid produced. Coulometric titration The main compartment of the titration cell contains the anode solution plus the analyte. The anode solution consists of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran. ( Furfural is produced from bran.) The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Lim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |