|
Tiamulin
Tiamulin (previously thiamutilin) is a pleuromutilin antibiotic drug that is used in veterinary medicine particularly for pigs and poultry. Tiamulin is a diterpene antimicrobial with a pleuromutilin chemical structure similar to that of valnemulin Valnemulin (trade name Econor or Biotilina) is a pleuromutilin antibiotic used to treat swine dysentery, ileitis, colitis and pneumonia. It is also used for the prevention of intestinal The gastrointestinal tract (GI tract, digestive tract, a .... References Pleuromutilin antibiotics Secondary alcohols Ketones Carboxylate esters Thioethers Cyclopentanes Diethylamino compounds Vinyl compounds {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
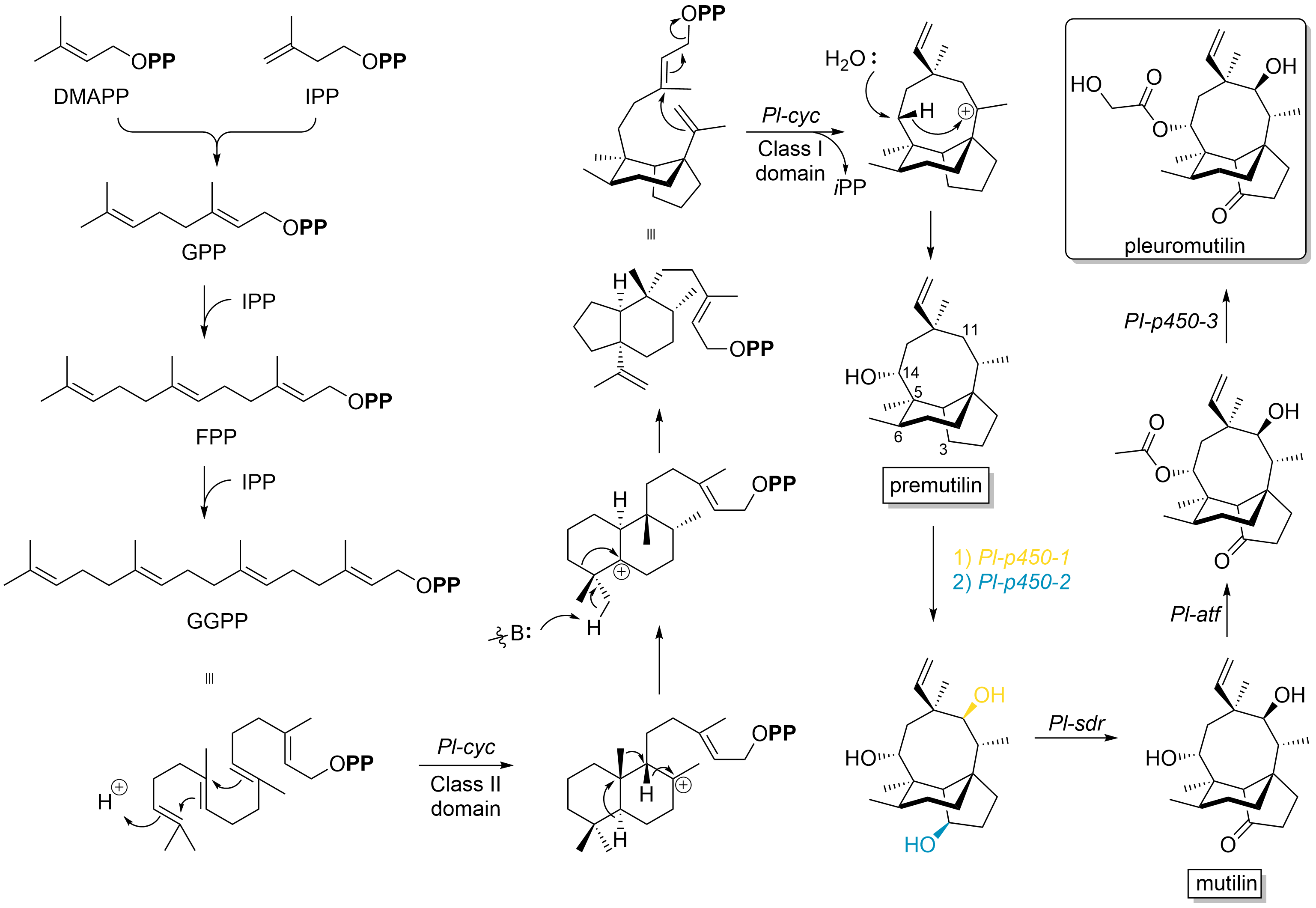
Pleuromutilin
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin. History Pleuromutilin was discovered as an antibiotic in 1951. It is derived from the fungi ''Omphalina mutila'' (formerly ''Pleurotus mutilus'') and ''Clitopilus passeckerianus'' (formerly ''Pleurotus passeckerianus''), and has also been found in ''Drosophila subatrata'', ''Clitopilus scyphoides'', and some other ''Clitopilus'' species. Total synthesis The total synthesis of pleuromutilin has been reported. Biosynthesis Pleuromutilin belongs to the class of secondary metabolites known as terpenes, which are produced in fungi through the mevalonate pathway (MEP pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleuromutilin Antibiotics
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin. History Pleuromutilin was discovered as an antibiotic in 1951. It is derived from the fungi '' Omphalina mutila'' (formerly ''Pleurotus mutilus'') and '' Clitopilus passeckerianus'' (formerly ''Pleurotus passeckerianus''), and has also been found in ''Drosophila subatrata'', ''Clitopilus scyphoides'', and some other '' Clitopilus'' species. Total synthesis The total synthesis of pleuromutilin has been reported. Biosynthesis Pleuromutilin belongs to the class of secondary metabolites known as terpenes, which are produced in fungi through the mevalonate path ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorgani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpene
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valnemulin
Valnemulin (trade name Econor or Biotilina) is a pleuromutilin antibiotic used to treat swine dysentery, ileitis, colitis and pneumonia. It is also used for the prevention of intestinal infections of swine. Valnemulin has been observed to induce a rapid reduction of clinical symptoms of ''Mycoplasma ''Mycoplasma'' is a genus of bacteria that, like the other members of the class '' Mollicutes'', lack a cell wall around their cell membranes. Peptidoglycan ( murein) is absent. This characteristic makes them naturally resistant to antibiotic ... bovis'' infection, and eliminate ''M. bovis'' from the lungs of calves. References Pleuromutilin antibiotics Secondary alcohols Ketones Thioethers Carboxamides Carboxylate esters Vinyl compounds {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate Esters
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylate esters'' have the general formula (or ). R and R′ are organic groups; R′ ≠ H. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. :RCOOH + NaOH -> RCOONa + H2O Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioethers
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentanes
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more rings of carbon atoms. It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure. It was first prepared in 1893 by the German chemist Johannes Wislicenus. Production, occurrence and use Cycloalkanes are formed by catalytic reforming. For example, when passed over a hot platinum surfact, 2-methylbutane converts into cyclopentane. Cyclopentane has no particular use. No commercial products are made from cyclopentane itself. As a volatile hydrocarbon it is an incidental component of some fuels and blowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |