|
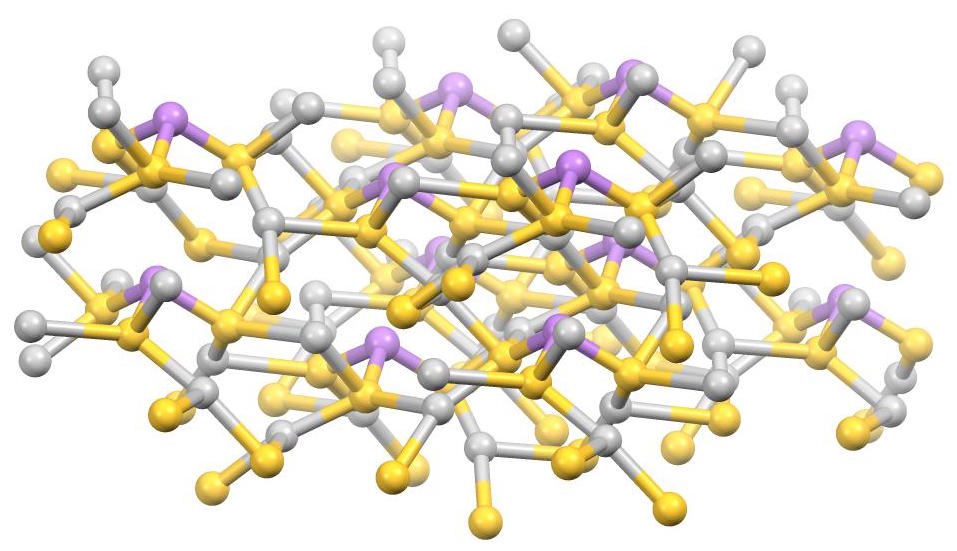
Tennantite
Tennantite is a copper arsenic sulfosalt mineral with an ideal formula . Due to variable substitution of the copper by iron and zinc the formula is . It is gray-black, steel-gray, iron-gray or black in color. A closely related mineral, tetrahedrite ) has antimony substituting for arsenic and the two form a solid solution series. The two have very similar properties and is often difficult to distinguish between tennantite and tetrahedrite. Iron, zinc, and silver substitute up to about 15% for the copper site. The mineral was first described for an occurrence in Cornwall, England in 1819, where it occurs as small crystals of cubic or dodecahedral form, and was named after the English chemist Smithson Tennant (1761–1815). It is found in hydrothermal veins and contact metamorphic deposits in association with other Cu–Pb–Zn–Ag sulfides and sulfosalts, pyrite, calcite, dolomite, siderite, barite, fluorite and quartz. The arsenic component of tennantite causes the metal smel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfosalt Minerals
Sulfosalt minerals are sulfide minerals with the general formula , where *A represents a metal such as copper, lead, silver, iron, and rarely mercury, zinc, vanadium *B usually represents semi-metal such as arsenic, antimony, bismuth, and rarely germanium, or metals like tin and rarely vanadium *X is sulfur or rarely selenium and/or tellurium. The Strunz classification includes the sulfosalts in a ''sulfides and sulfosalts'' superclass. A group which have similar appearing formulas are the sulfarsenides (for example cobaltite (Co,Fe)AsS). In sulfarsenides the arsenic substitutes for sulfide anions whereas in the sulfosalts the arsenic substitutes for a metal cation.Klein, Cornelis and Cornelius S. Hurlbut (1985). ''Manual of Mineralogy'', 20th ed., John Wiley and Sons, New York . About 200 sulfosalt minerals are known. Examples include: File:Proustite (long prismatic crystal) - Chanarcillo, Copiapo Province, Atacama Region, Chile.jpg, As illustrated by this specimen of pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfosalt Mineral
Sulfosalt minerals are sulfide minerals with the general formula , where *A represents a metal such as copper, lead, silver, iron, and rarely mercury, zinc, vanadium *B usually represents semi-metal such as arsenic, antimony, bismuth, and rarely germanium, or metals like tin and rarely vanadium *X is sulfur or rarely selenium and/or tellurium. The Strunz classification includes the sulfosalts in a ''sulfides and sulfosalts'' superclass. A group which have similar appearing formulas are the sulfarsenides (for example cobaltite (Co,Fe)AsS). In sulfarsenides the arsenic substitutes for sulfide anions whereas in the sulfosalts the arsenic substitutes for a metal cation.Klein, Cornelis and Cornelius S. Hurlbut (1985). ''Manual of Mineralogy'', 20th ed., John Wiley and Sons, New York . About 200 sulfosalt minerals are known. Examples include: File:Proustite (long prismatic crystal) - Chanarcillo, Copiapo Province, Atacama Region, Chile.jpg, As illustrated by this specimen o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedrite
Tetrahedrite is a copper antimony sulfosalt mineral with formula: . It is the antimony endmember of the continuous solid solution series with arsenic-bearing tennantite. Pure endmembers of the series are seldom if ever seen in nature. Of the two, the antimony rich phase is more common. Other elements also substitute in the structure, most notably iron and zinc, along with less common silver, mercury and lead. Bismuth also substitutes for the antimony site and ''bismuthian tetrahedrite'' or ''annivite'' is a recognized variety. The related, silver dominant, mineral species freibergite, although rare, is notable in that it can contain up to 18% silver. Mineralogy Tetrahedrite gets its name from the distinctive tetrahedron shaped cubic crystals. The mineral usually occurs in massive form, it is a steel gray to black metallic mineral with Mohs hardness of 3.5 to 4 and specific gravity of 4.6 to 5.2. Tetrahedrite occurs in low to moderate temperature hydrothermal veins and in so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smithson Tennant
Smithson Tennant FRS (30 November 1761 – 22 February 1815) was an English chemist. He is best known for his discovery of the elements iridium and osmium, which he found in the residues from the solution of platinum ores in 1803. He also contributed to the proof of the identity of diamond and charcoal. The mineral tennantite is named after him. Life Tennant was born in Selby in Yorkshire. His father was Calvert Tennant (named after his grandmother Phyllis Calvert, a granddaughter of Cecilius Calvert, 2nd Baron Baltimore). His own name derives from his grandmother Rebecca Smithson, widow of Joshua Hitchling. He attended Beverley Grammar School and there is a plaque over one of the entrances to the present school commemorating his discovery of the two elements, osmium and iridium. He began to study medicine at Edinburgh in 1781, but after a few months moved to Cambridge, where he devoted himself to botany and chemistry. He graduated M.D. at Cambridge in 1796, and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form (native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III-V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in rats, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contact Metamorphism
Metamorphism is the transformation of existing rock (the protolith) to rock with a different mineral composition or texture. Metamorphism takes place at temperatures in excess of , and often also at elevated pressure or in the presence of chemically active fluids, but the rock remains mostly solid during the transformation. Metamorphism is distinct from weathering or diagenesis, which are changes that take place at or just beneath Earth's surface. Various forms of metamorphism exist, including regional, contact, hydrothermal, shock, and dynamic metamorphism. These differ in the characteristic temperatures, pressures, and rate at which they take place and in the extent to which reactive fluids are involved. Metamorphism occurring at increasing pressure and temperature conditions is known as ''prograde metamorphism'', while decreasing temperature and pressure characterize ''retrograde metamorphism''. Metamorphic petrology is the study of metamorphism. Metamorphic petrologists re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, opacity, and luster, but may have properties that differ from those of the pure metals, such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength. Alloys are defined by a metallic bonding character. The alloy constituents are usually measured by mass percentage for practical applications, and in atomic fraction for basic science studies. Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy. They can be further classified as homo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smelting
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gases or slag and leaving the metal base behind. The reducing agent is commonly a fossil fuel source of carbon, such as coke—or, in earlier times, charcoal. The oxygen in the ore binds to carbon at high temperatures due to the lower potential energy of the bonds in carbon dioxide (). Smelting most prominently takes place in a blast furnace to produce pig iron, which is converted into steel. The carbon source acts as a chemical reactant to remove oxygen from the ore, yielding the purified metal element as a product. The carbon source is oxidized in two stages. First, the carbon (C) combusts with oxygen (O2) in the air to produce carbon monoxide (CO). Seco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar. Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at . Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold. There are many different varieties of quartz, several of which are classified as gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia. Quartz is the mineral definin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
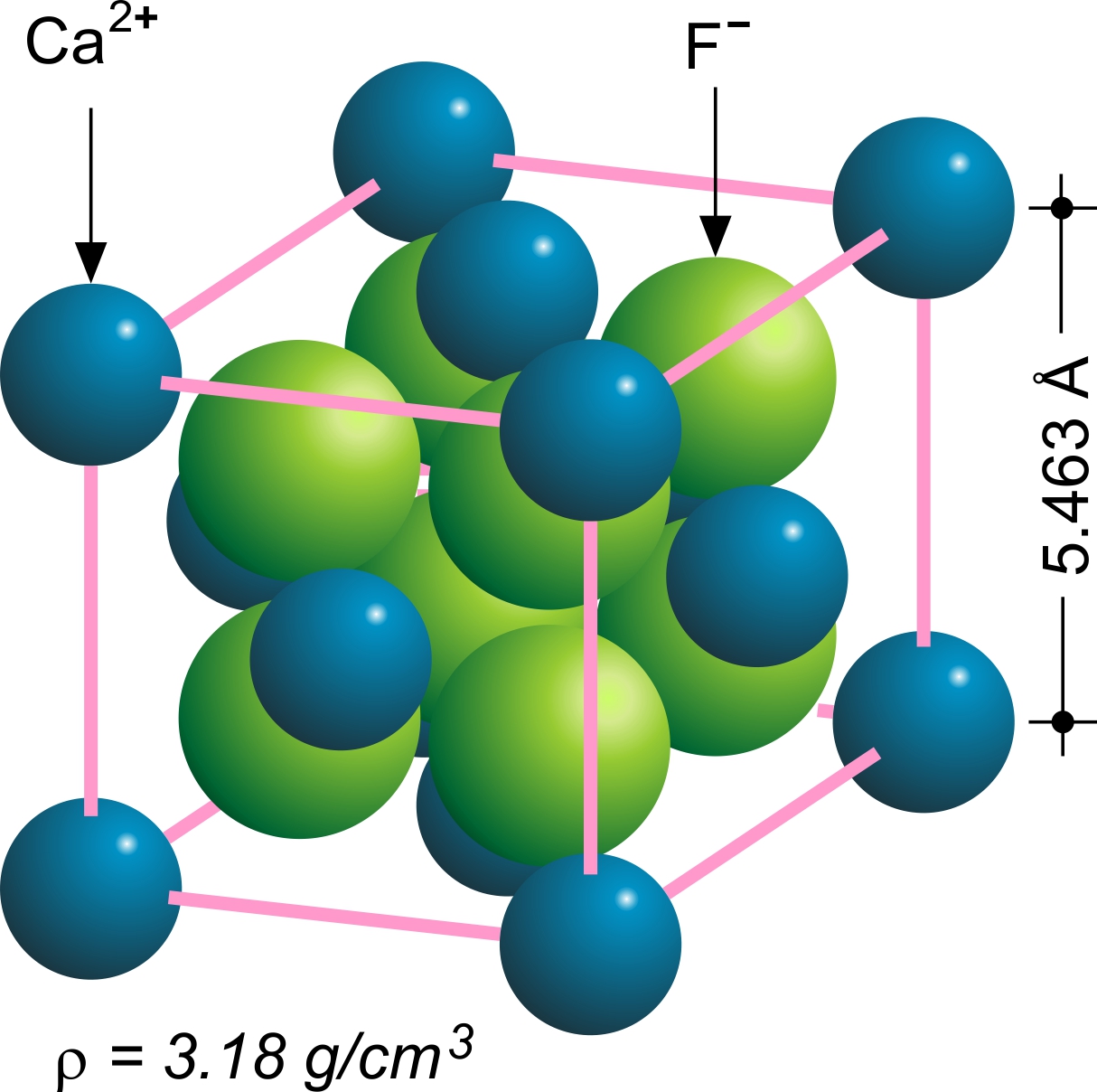
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |