|
Strain Energy
In physics, the elastic potential energy gained by a wire during elongation with a tensile (stretching) force is called strain energy. For Linear elasticity, linearly elastic materials, strain energy is: : U = \frac 1 2 V \sigma \epsilon = \frac 1 2 V E \epsilon^2 = \frac 1 2 \frac V E \sigma^2 where is Stress (mechanics), stress, is Deformation (engineering), strain, is volume, and is Young's modulus: : E = \frac \sigma \epsilon Molecular strain In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction.''March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure,'' Michael B. Smith & Jerry March, Wiley-Interscience, 5th edition, 2001, The external work done on an elastic member in causing it to distort from its unstressed state is transformed into strain energy which is a form of potential energy. The strain energy in the form of elastic deformation is mostly recoverable in the form of mec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." Physics is one of the most fundamental scientific disciplines, with its main goal being to understand how the universe behaves. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into cli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclophane
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit (typically a benzene ring) and a chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied examples of strained organic compounds. Cyclophanes Structures Paracyclophanes adopt the boat conformation normally observed in cyclohexanes. Smaller value of n lead to greater distortions. X-ray crystallography on ' aracyclophane' shows that the aromatic bridgehead carbon atom makes an angle of 20.5° with the plane. The benzyl carbons deviate by another 20.2°. The carbon-to-carbon bond length alternation has increased from 0 for benzene to 39 pm. Despite their distorted structures, cyclophanes retain their aromaticity, as determined by UV-vis spectroscopy. Reactivity With regards to their reactivity, cyclophanes often exhibit diene-like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fenestrane
A fenestrane in organic chemistry is a type of chemical compound with a central quaternary carbon atom which serves as a common vertex for four fused carbocycles. They can be regarded as spiro compounds twice over. Because of their inherent strain and instability, fenestranes are of theoretical interest to chemists. The name—proposed in 1972 by Vlasios Georgian and Martin Saltzman—is derived from the Latin word for window, ''fenestra''. Georgian had intended that "fenestrane" solely referred to .4.4.4enestrane, whose skeletal structure looks like windows, and Kenneth B. Wiberg called that specific structure "windowpane". The term ''fenestrane'' has since become generalized to refer to the whole class of molecules that have various other ring-sizes. Georgian recommended ''rosettane'' for the class, based on the structural appearance as a rosette of flowers. Nomenclature and structure Structures within this class of chemicals can be named according to the number of atoms in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubane-type Cluster
A cubane-type cluster is an arrangement of atoms in a molecular structure that forms a cube. In the idealized case, the eight vertices are symmetry equivalent and the species has Oh symmetry. Such a structure is illustrated by the hydrocarbon cubane. With chemical formula , cubane has carbon atoms at the corners of a cube and covalent bonds forming the edges. Most cubanes have more complicated structures, usually with nonequivalent vertices. They may be simple covalent compounds or macromolecular or supramolecular cluster compounds. Examples Other compounds having different elements in the corners, various atoms or groups bonded to the corners are all part of this class of structures. Inorganic cubane-type clusters include selenium tetrachloride, tellurium tetrachloride, and sodium silox. Cubane clusters are common throughout bioinorganic chemistry. Ferredoxins containing e4S4iron–sulfur clusters are pervasive in nature. The four iron atoms and four sulfur atoms form an alter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propellane
In organic chemistry, propellane is any member of a class of polycyclic hydrocarbons, whose carbon skeleton consists of three rings of carbon atoms sharing a common carbon–carbon covalent bond. The concept was introduced in 1966 by D. Ginsburg Propellanes with small cycles are highly strained and unstable, and are easily turned into polymers with interesting structures, such as staffanes. Partly for these reasons, they have been the object of much research. Nomenclature The name derives from a supposed resemblance of the molecule to a propeller: namely, the rings would be the propeller's blades, and the shared C–C bond would be its axis. The bond shared by the three cycles is usually called the "bridge"; the shared carbon atoms are then the "bridgeheads". The IUPAC nomenclature of the homologue series of all-carbon propellanes would be called tricyclo .y.z.01,(x+2)lkane. More common in literature is the notation means the member of the family whose rings have ''x'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
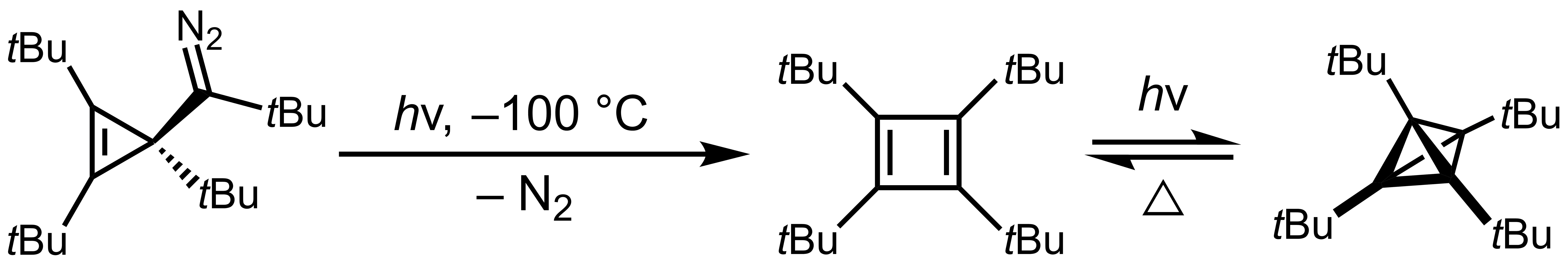
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. Organic tetrahedranes In 1978, Günther Maier prepared tetra- ''tert''-butyl-tetrahedrane. These bulky substituents envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain. Tetrahedrane is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane () remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of the molecule. It is the repeating unit in the skeleton of the unbranched alkanes. A methylene bridge can also act as a bidentate ligand joining two metals in a coordination compound, such as titanium and aluminum in Tebbe's reagent.W. A. Herrmann (1982), "The methylene bridge". In ''Advances in Organometallic Chemistry'', volume 20, pages 195-197. A methylene bridge is often called a methylene group or simply methylene, as in "methylene chloride" (dichloromethane ). As a bridge in other compounds, for example in cyclic compounds, it is given the name methano. However, the term methylene group (or "methylidene") properly applies to the group when it is connected to the rest of the molecule by a double bond (), giving it chemical proper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases (LP gases). The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal. Propane gas has become a popular choice for barbecues and portable stoves because its low −42 °C boiling point makes it vaporise inside pressurised liquid containers (2 phases). Propane powers buses, forklifts, taxis, outboard boat motors, and ic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Of Combustion
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it. The ''calorific value'' is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon or other organic molecule reacting with oxygen to form carbon dioxide and water and release heat. It may be expressed with the quantities: * energy/mole of fuel * energy/mass of fuel * energy/volume of the fuel There are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much the products are allowed to cool and whether compounds like are allowed to condense. The high heat values are conventionally measured with a bomb calorimeter. Low heat values are calculated from high heat value test data. They may also be calculated as the difference betw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastic Potential Energy
Elastic energy is the mechanical potential energy stored in the configuration of a material or physical system as it is subjected to elastic deformation by work performed upon it. Elastic energy occurs when objects are impermanently compressed, stretched or generally deformed in any manner. Elasticity theory primarily develops formalisms for the mechanics of solid bodies and materials. (Note however, the work done by a stretched rubber band is not an example of elastic energy. It is an example of entropic elasticity.) The elastic potential energy equation is used in calculations of positions of mechanical equilibrium. The energy is potential as it will be converted into other forms of energy, such as kinetic energy and sound energy, when the object is allowed to return to its original shape (reformation) by its elasticity. U = \frac 1 2 k\, \Delta x^2 The essence of elasticity is reversibility. Forces applied to an elastic material transfer energy into the material which, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |