Fenestrane on:
[Wikipedia]
[Google]
[Amazon]
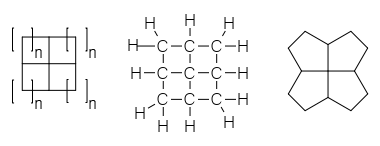 A fenestrane in
A fenestrane in

 Structures within this class of chemicals can be named according to the number of atoms in each ring in addition to the systematic nomenclature of IUPAC naming rules. The smallest member of the family, consisting of four fused cyclopropane rings, is .3.3.3enestrane, which has systematic name tetracyclo .1.0.01,3.02,5entane and is also called pyramidane. The next symmetric member, .4.4.4enestrane, has four
Structures within this class of chemicals can be named according to the number of atoms in each ring in addition to the systematic nomenclature of IUPAC naming rules. The smallest member of the family, consisting of four fused cyclopropane rings, is .3.3.3enestrane, which has systematic name tetracyclo .1.0.01,3.02,5entane and is also called pyramidane. The next symmetric member, .4.4.4enestrane, has four  In addition to the ring sizes, fenestranes can have various combinations of cis and trans geometry at each ring fusion. These details are denoted by "''c''" and "''t''" prefixes to the structure name, listed in the same order as the ring-sizes. For example, ''c'',''t'',''c'',''c''- .5.5.5enestrane has a trans configuration at one of the cyclopentane/cyclopentane fusions, but cis configuration at the other cyclopentane/cyclopentane fusion and at both butanepentane/cyclopentane fusions.
In an extreme case the central carbon atom, which would ordinarily have tetrahedral molecular geometry for its four bonds gets completely flattened. In the
In addition to the ring sizes, fenestranes can have various combinations of cis and trans geometry at each ring fusion. These details are denoted by "''c''" and "''t''" prefixes to the structure name, listed in the same order as the ring-sizes. For example, ''c'',''t'',''c'',''c''- .5.5.5enestrane has a trans configuration at one of the cyclopentane/cyclopentane fusions, but cis configuration at the other cyclopentane/cyclopentane fusion and at both butanepentane/cyclopentane fusions.
In an extreme case the central carbon atom, which would ordinarily have tetrahedral molecular geometry for its four bonds gets completely flattened. In the 
 In the
In the 
 A fenestrane in
A fenestrane in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
is a type of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one ele ...
with a central quaternary carbon atom which serves as a common vertex for four fused carbocycles. They can be regarded as spiro compound
In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic (having just two rings), or have a bicyclic portion as part of the larger ring sy ...
s twice over. Because of their inherent strain and instability, fenestranes are of theoretical interest to chemists. The name—proposed in 1972 by Vlasios Georgian and Martin Saltzman—is derived from the Latin
Latin (, or , ) is a classical language belonging to the Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power ...
word for window
A window is an opening in a wall, door, roof, or vehicle that allows the exchange of light and may also allow the passage of sound and sometimes air. Modern windows are usually glazed or covered in some other transparent or translucent mate ...
, ''fenestra''. Georgian had intended that "fenestrane" solely referred to .4.4.4enestrane, whose skeletal structure looks like windows, and Kenneth B. Wiberg called that specific structure "windowpane". The term ''fenestrane'' has since become generalized to refer to the whole class of molecules that have various other ring-sizes. Georgian recommended ''rosettane'' for the class, based on the structural appearance as a rosette of flowers.
Nomenclature and structure

 Structures within this class of chemicals can be named according to the number of atoms in each ring in addition to the systematic nomenclature of IUPAC naming rules. The smallest member of the family, consisting of four fused cyclopropane rings, is .3.3.3enestrane, which has systematic name tetracyclo .1.0.01,3.02,5entane and is also called pyramidane. The next symmetric member, .4.4.4enestrane, has four
Structures within this class of chemicals can be named according to the number of atoms in each ring in addition to the systematic nomenclature of IUPAC naming rules. The smallest member of the family, consisting of four fused cyclopropane rings, is .3.3.3enestrane, which has systematic name tetracyclo .1.0.01,3.02,5entane and is also called pyramidane. The next symmetric member, .4.4.4enestrane, has four cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercia ...
rings fused, and has systematic name tetracyclo .3.1.03,9.07,9onane. The rings need not all be the same size as each other, so .4.4.5enestrane has three cyclobutane rings and one cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It oc ...
ring. Other structural modifications vary the name as usual in systematic nomenclature, so a .6.4.6enestradiene has two cyclobutane rings and two cyclohexane rings in an alternating pattern and two alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
units in the ring structure.
 In addition to the ring sizes, fenestranes can have various combinations of cis and trans geometry at each ring fusion. These details are denoted by "''c''" and "''t''" prefixes to the structure name, listed in the same order as the ring-sizes. For example, ''c'',''t'',''c'',''c''- .5.5.5enestrane has a trans configuration at one of the cyclopentane/cyclopentane fusions, but cis configuration at the other cyclopentane/cyclopentane fusion and at both butanepentane/cyclopentane fusions.
In an extreme case the central carbon atom, which would ordinarily have tetrahedral molecular geometry for its four bonds gets completely flattened. In the
In addition to the ring sizes, fenestranes can have various combinations of cis and trans geometry at each ring fusion. These details are denoted by "''c''" and "''t''" prefixes to the structure name, listed in the same order as the ring-sizes. For example, ''c'',''t'',''c'',''c''- .5.5.5enestrane has a trans configuration at one of the cyclopentane/cyclopentane fusions, but cis configuration at the other cyclopentane/cyclopentane fusion and at both butanepentane/cyclopentane fusions.
In an extreme case the central carbon atom, which would ordinarily have tetrahedral molecular geometry for its four bonds gets completely flattened. In the molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findi ...
picture for the resulting square planar geometry of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
, two of a total of three sp2-hybridized carbon atomic orbitals form regular bonds with two of the hydrogen atoms as in a planar alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
. The third sp2 orbital interacts in a three-center two-electron bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''- ...
with the two remaining hydrogen atoms utilizing only the hydrogen electrons. Two additional carbon valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair f ...
s are situated in a p orbital perpendicular to the plane of the molecule. The four C–H bonds are equal due to resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
. In silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on computer or via computer simulation. The phrase is pseudo-Latin for 'in silicon' (correct la, in silicio), referring to silicon in computer chips. It ...
calculations show that it takes 95 to 250 kcal/mol (400 to 1,050 kJ/mol) for this process.
One of the most highly strained fenestranes to have been isolated is a .4.4.5enestrane with bond angles at the central carbon atom of around 130° (based on X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angle ...
), as compared to the 109.45° standard for tetrahedral atoms. The carbon–carbon bond-lengths deviate from those of normal alkanes as well. Whereas the C–C bond in ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petro ...
is 155 pm, in this fenestrane, the bonds extending from the central carbon atom are shortened to 149 pm while those at the perimeter are lengthened to 159 pm.
A diterpene called laurenene
Laurenene is a diterpene natural product with an unusual .5.5.7 enestrane structure. It was first discovered in extracts from the New Zealand tree species ''Dacrydium cupressinum'' by researchers at the University of Otago
, image_name = Un ...
containing a .5.5.7enestrane ring system was the first natural fenestrane to be discovered. The first fenestrane ever synthesized was a .5.5.6enestrane:

Pyramidanes
Pyramidane ( .3.3.3enestrane) is the smallest possible fenestrane, and has never been synthesised. If the central carbon were to be tetrahedral, it would have the form of spiropentadiene, but with additional bonds between the two cyclopropyl rings rather than double-bonds within them. The analogous germa- and stannapyramidanes, withtrimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is c ...
groups bonded to the corners, Ge 4(SiMe3)4and Sn 4(SiMe3)4on the other hand have been synthesised. These adopt a square pyramidal geometry analogous to the trigonal pyramid of tetrahedrane, with the germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbo ...
or tin atom at the vertex. That atom has an inverted tetrahedral geometry. According to nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
analysis, the four carbons of the base of the pyramid behave as an aromatic ring
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satu ...
.
Synthetic approaches
In one study, a .5.5.5enestrane was synthesized with one carbon atom replaced by nitrogen becauseaza-
The prefix aza- is used in organic chemistry to form names of organic compounds where a carbon atom is replaced by a nitrogen atom. The related term "deaza-" refers to when a nitrogen is removed and, usually, a carbon atom is put in its place. ...
compounds and their salts are more likely to form crystalline compounds suitable for X-ray analysis than low-molecular-weight alkanes. In step 1 the alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
1-iodo-3-butene 1 is converted to a cyanozinc cuprate 2 (by transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
of the organozinc iodide with copper cyanide
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two Polymorphism (materials science), polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a c ...
) which reacts in the next step with 1-nitrocyclopentene 3 in a nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions ...
whereby the nitronate 4 is captured by phenylselenenyl bromide to the selenium intermediate 5. Hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3% ...
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of 5 yields the nitroalkene 6 as a mixture of ''syn'' and ''anti'' isomers. A +2 ycloaddition with ''n''-butylenol ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers includ ...
in presence of trimethylaluminium gives the nitronate 7 and a second +2ycloaddition by heating in presence of potassium carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and gl ...
gives the nitroso
In organic chemistry, nitroso refers to a functional group in which the nitric oxide () group is attached to an organic moiety. As such, various nitroso groups can be categorized as ''C''-nitroso compounds (e.g., nitrosoalkanes; ), ''S''-nitroso ...
acetal 8. Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
with Raney nickel gives the diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is ...
9 which on a double Mitsunobu reaction (with an amine proton donor) gives the azafenestrane 10 as the borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated ...
salt.
 In the
In the borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated ...
salt the N–C–C bond angle is 126°.
One study describes an unusual 8π disrotatory – 6π conrotatory electrocyclic cascade reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the p ...
aiming to minimise the number of steps required to synthesise a fenestrane.Reagents: P-2 Ni (Ni(OAc)2·4H2O) / hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
gas. Reaction initiated by organic reduction of alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
to alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
See also
* Acepentalene * TriquinaceneReferences
{{Reflist Polycyclic nonaromatic hydrocarbons