|
Spherocytes
Spherocytosis is the presence of spherocytes in the blood, i.e. erythrocytes (red blood cells) that are sphere-shaped rather than bi-concave disk shaped as normal. Spherocytes are found in all hemolytic anemias to some degree. Hereditary spherocytosis and autoimmune hemolytic anemia are characterized by having ''only'' spherocytes. Causes Spherocytes are found in immunologically-mediated hemolytic anemias and in hereditary spherocytosis, but the former would have a positive direct Coombs test and the latter would not. The misshapen but otherwise healthy red blood cells are mistaken by the spleen for old or damaged red blood cells and it thus constantly breaks them down, causing a cycle whereby the body destroys its own blood supply (auto-hemolysis). A complete blood count (CBC) may show increased reticulocytes, a sign of increased red blood cell production, and decreased hemoglobin and hematocrit. The term "non-hereditary spherocytosis" is occasionally used, albeit rarely. Lists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hereditary Spherocytosis
Hereditary spherocytosis (HS) is a congenital hemolytic disorder, wherein a genetic mutation coding for a structural membrane protein phenotype leads to a spherical shaping of erythrocytic cellular morphology. As erythrocytes are sphere-shaped (spherocytosis), rather than the normal biconcave disk-shaped, their morphology interferes with these cells' abilities to be flexible during circulation throughout the entirety of the body - arteries, arterioles, capillaries, venules, veins, and organs. This difference in shape also makes the red blood cells more prone to rupture under osmotic and/or mechanical stress. Cells with these dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes resulting in hemolytic anemia. HS was first described in 1871, and is the most common cause of inherited hemolysis in populations of northern European descent, with an incidence of 1 in 5000 births. The clinical severity of HS varies from mild (symptom-free carrier) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hereditary Spherocytosis
Hereditary spherocytosis (HS) is a congenital hemolytic disorder, wherein a genetic mutation coding for a structural membrane protein phenotype leads to a spherical shaping of erythrocytic cellular morphology. As erythrocytes are sphere-shaped (spherocytosis), rather than the normal biconcave disk-shaped, their morphology interferes with these cells' abilities to be flexible during circulation throughout the entirety of the body - arteries, arterioles, capillaries, venules, veins, and organs. This difference in shape also makes the red blood cells more prone to rupture under osmotic and/or mechanical stress. Cells with these dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes resulting in hemolytic anemia. HS was first described in 1871, and is the most common cause of inherited hemolysis in populations of northern European descent, with an incidence of 1 in 5000 births. The clinical severity of HS varies from mild (symptom-free carrier) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemolytic Anemia
Hemolytic anemia or haemolytic anaemia is a form of anemia due to hemolysis, the abnormal breakdown of red blood cells (RBCs), either in the blood vessels (intravascular hemolysis) or elsewhere in the human body (extravascular). This most commonly occurs within the spleen, but also can occur in the reticuloendothelial system or mechanically (prosthetic valve damage). Hemolytic anemia accounts for 5% of all existing anemias. It has numerous possible consequences, ranging from general symptoms to life-threatening systemic effects. The general classification of hemolytic anemia is either intrinsic or extrinsic. Treatment depends on the type and cause of the hemolytic anemia. Symptoms of hemolytic anemia are similar to other forms of anemia (fatigue and shortness of breath), but in addition, the breakdown of red cells leads to jaundice and increases the risk of particular long-term complications, such as gallstones and pulmonary hypertension. Signs and symptoms Symptoms of hemolytic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements. A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrin
Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
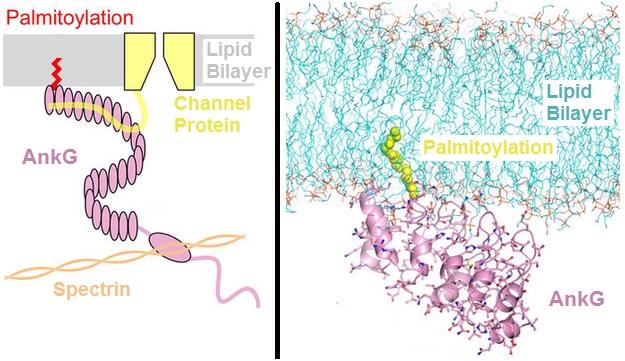
Ankyrin
Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the plasma membranes and to anchor specific ion channels, ion exchangers and ion transporters in the plasma membrane. The name is derived from the Greek word ἄγκυρα (''ankyra'') for "anchor". Structure Ankyrins contain four functional domains: an N-terminal domain that contains 24 tandem ankyrin repeats, a central domain that binds to spectrin, a death domain that binds to proteins involved in apoptosis, and a C-terminal regulatory domain that is highly variable between different ankyrin proteins. Membrane protein recognition The 24 tandem ankyrin repeats are responsible for the recognition of a wide range of membrane proteins. These 24 repeats contain 3 str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band 3
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the gene in humans. Band 3 anion transport protein is a phylogenetically-preserved transport protein responsible for mediating the exchange of chloride (Cl−) with bicarbonate (HCO3−) across plasma membranes. Functionally similar members of the AE clade are AE2 and AE3. Function Band 3 is present in the basolateral face of the α-intercalated cells of the collecting ducts of the nephron, which are the main acid-secreting cells of the kidney. They generate hydrogen ions and bicarbonate ions from carbon dioxide and water – a reaction catalysed by carbonic anhydrase. The hydrogen ions are pumped into the collecting duct tubule by vacuolar H+ ATPase, the apical proton pump, which thus excretes acid into the urine. kAE1 exchanges bicarbonate for chloride on the basolateral surface, essentially returning bicarbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein 4
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |