|
Ankyrin
Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the plasma membranes and to anchor specific ion channels, ion exchangers and ion transporters in the plasma membrane. The name is derived from the Greek word ἄγκυρα (''ankyra'') for "anchor". Structure Ankyrins contain four functional domains: an N-terminal domain that contains 24 tandem ankyrin repeats, a central domain that binds to spectrin, a death domain that binds to proteins involved in apoptosis, and a C-terminal regulatory domain that is highly variable between different ankyrin proteins. Membrane protein recognition The 24 tandem ankyrin repeats are responsible for the recognition of a wide range of membrane proteins. These 24 repeats contain 3 struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ANK2
Ankyrin-2, also known as Ankyrin-B, and Brain ankyrin, is a protein which in humans is encoded by the ''ANK2'' gene. Ankyrin-2 is ubiquitously expressed, but shows high expression in cardiac muscle. Ankyrin-2 plays an essential role in the localization and membrane stabilization of ion transporters and ion channels in cardiomyocytes, as well as in costamere structures. Mutations in ''ANK2'' cause a dominantly-inherited, cardiac arrhythmia syndrome known as long QT syndrome 4 as well as sick sinus syndrome; mutations have also been associated to a lesser degree with hypertrophic cardiomyopathy. Alterations in ankyrin-2 expression levels are observed in human heart failure. Structure Ankyrin-B protein is around 220 kDa, with several isoforms. The ''ANK2'' gene is approximately 560 kb in size and consists of 53 exons on human chromosome 4; ''ANK2'' is also transcriptionally regulated via over 30 alternative splicing events with variable expression of isoforms in cardiac muscle. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hereditary Spherocytosis
Hereditary spherocytosis (HS) is a congenital hemolytic disorder, wherein a genetic mutation coding for a structural membrane protein phenotype leads to a spherical shaping of erythrocytic cellular morphology. As erythrocytes are sphere-shaped (spherocytosis), rather than the normal biconcave disk-shaped, their morphology interferes with these cells' abilities to be flexible during circulation throughout the entirety of the body - arteries, arterioles, capillaries, venules, veins, and organs. This difference in shape also makes the red blood cells more prone to rupture under osmotic and/or mechanical stress. Cells with these dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes resulting in hemolytic anemia. HS was first described in 1871, and is the most common cause of inherited hemolysis in populations of northern European descent, with an incidence of 1 in 5000 births. The clinical severity of HS varies from mild (symptom-free carrier), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
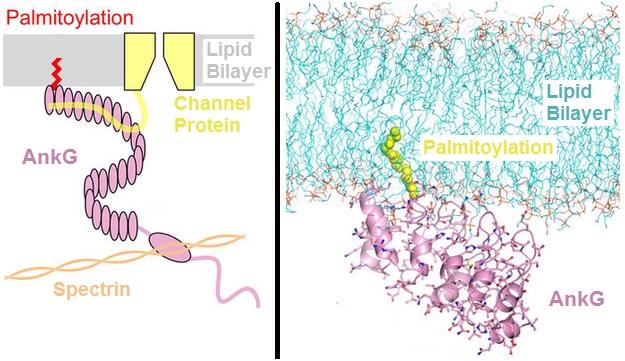
ANK3
Ankyrin-3 (ANK-3), also known as ankyrin-G, is a protein from ankyrin family that in humans is encoded by the ''ANK3'' gene. Function The protein encoded by this gene, ankyrin-3 is an immunologically distinct gene product from ankyrins ANK1 and ANK2, and was originally found at the axonal initial segment and nodes of Ranvier of neurons in the central and peripheral nervous systems. Alternatively spliced variants may be expressed in other tissues. Although multiple transcript variants encoding several different isoforms have been found for this gene, the full-length nature of only two have been characterized. Within the nervous system, ankyrin-G is specifically localized to the neuromuscular junction, the axon initial segment and the Nodes of Ranvier. Within the nodes of Ranvier where action potentials are actively propagated, ankyrin-G has long been thought to be the intermediate binding partner to neurofascin and voltage-gated sodium channels. The genetic deletion of ankyrin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ankyrin Repeat
The ankyrin repeat is a 33-residue motif in proteins consisting of two alpha helices separated by loops, first discovered in signaling proteins in yeast Cdc10 and ''Drosophila'' Notch. Domains consisting of ankyrin tandem repeats mediate protein–protein interactions and are among the most common structural motifs in known proteins. They appear in bacterial, archaeal, and eukaryotic proteins, but are far more common in eukaryotes. Ankyrin repeat proteins, though absent in most viruses, are common among poxviruses. Most proteins that contain the motif have four to six repeats, although its namesake ankyrin contains 24, and the largest known number of repeats is 34, predicted in a protein expressed by ''Giardia lamblia''. Ankyrin repeats typically fold together to form a single, linear solenoid structure called ankyrin repeat domains. These domains are one of the most common protein–protein interaction platforms in nature. They occur in a large number of functionally diverse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ANK1
Ankyrin 1, also known as ANK-1, and erythrocyte ankyrin, is a protein that in humans is encoded by the ''ANK1'' gene. Tissue distribution The protein encoded by this gene, Ankyrin 1, is the prototype of the ankyrin family, was first discovered in erythrocytes, but since has also been found in brain and muscles. Genetics Complex patterns of alternative splicing in the regulatory domain, giving rise to different isoforms of ankyrin 1 have been described, however, the precise functions of the various isoforms are not known. Alternative polyadenylation accounting for the different sized erythrocytic ankyrin 1 mRNAs, has also been reported. Truncated muscle-specific isoforms of ankyrin 1 resulting from usage of an alternate promoter have also been identified. Disease linkage Mutations in erythrocytic ankyrin 1 have been associated in approximately half of all patients with hereditary spherocytosis. ANK1 shows altered methylation and expression in Alzheimer's disease. A gene expre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KAHRP
KAHRP (knob-associated histidine-rich protein) is a protein expressed by ''Plasmodium falciparum'' infecting erythrocytes. KAHRP is a major component of knobs, feature found on Plasmodium falciparum infected erythrocytes. It has been suggested that KAHRP may play a role in trafficking or docking PfEMP1, major malarial cytoadherence protein to the erythrocyte membrane; however, these findings were disputed by recent NMR and fluorescence anisotropy studies showing no interaction between PfEMP1 and KAHRP. Instead, KAHRP was shown to interact with Ankyrin, more precisely the D3 subunit of the Membrane-binding domain of Ankyrin type 1. This interaction was suggested via SPR, ELISA, and Pulldown studies, however, it has not been confirmed by NMR, ITC, crystallography Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (conde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DARPin
DARPins (an acronym for designed ankyrin repeat proteins) are genetically engineered antibody mimetic proteins typically exhibiting highly specific and high-affinity target protein binding. They are derived from natural ankyrin repeat proteins, one of the most common classes of binding proteins in nature, which are responsible for diverse functions such as cell signaling, regulation and structural integrity of the cell. DARPins consist of at least three, repeat motifs or modules, of which the most N- and the most C-terminal modules are referred to as "caps", since they shield the hydrophobic core of the protein. The number of internal modules is indicated as number (e.g. N1C, N2C, N3C, ...) while the caps are indicated with "N" or "C", respectively. The molecular mass of e.g. 14 or 18 kDa (kilodaltons) for four- (N2C) or five- (N3C) repeat DARPins is rather small for a biologic (ca 10% of the size of an IgG). DARPins constitute a new class of potent, specific and versatile sma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
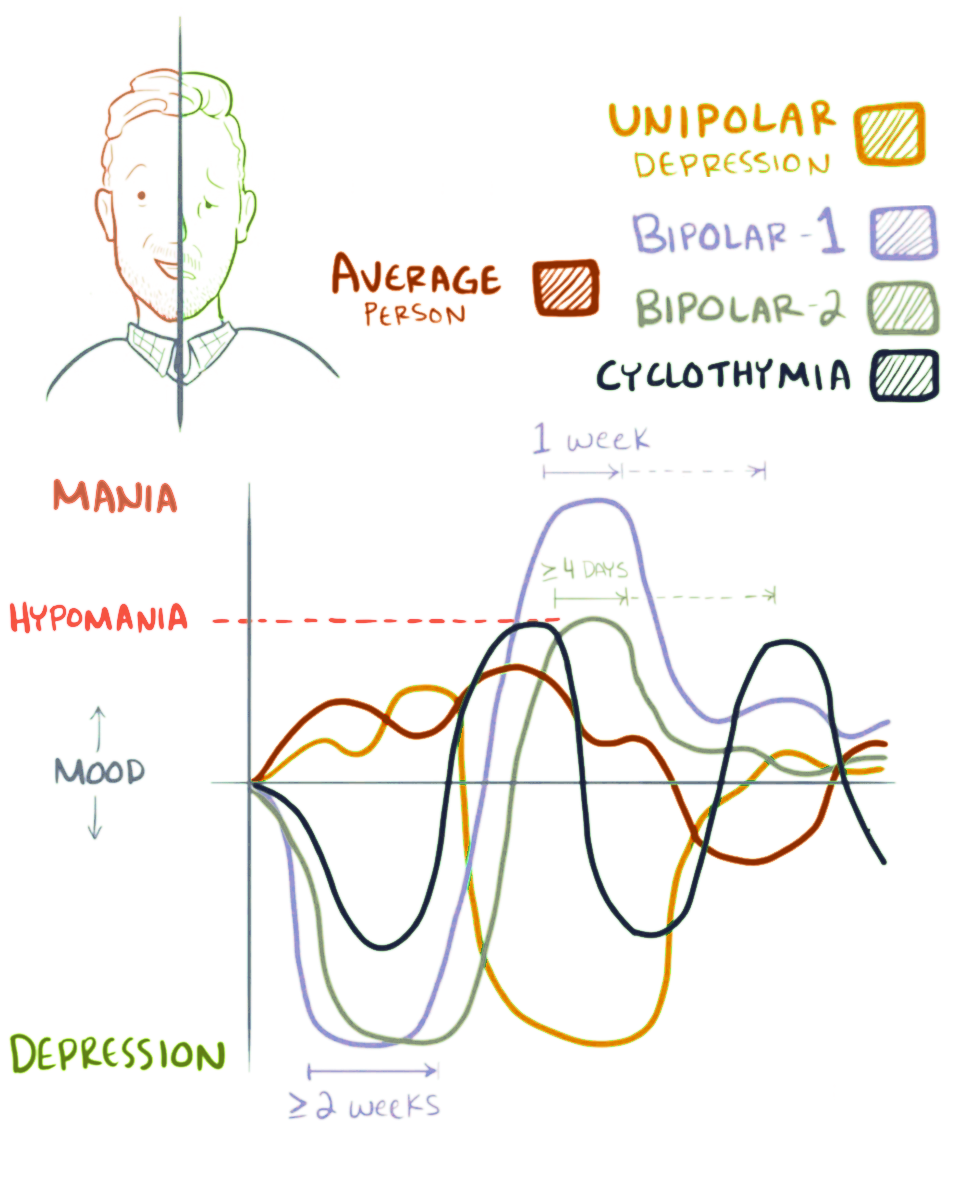
Bipolar Disorder
Bipolar disorder, previously known as manic depression, is a mental disorder characterized by periods of depression and periods of abnormally elevated mood that last from days to weeks each. If the elevated mood is severe or associated with psychosis, it is called mania; if it is less severe, it is called hypomania. During mania, an individual behaves or feels abnormally energetic, happy or irritable, and they often make impulsive decisions with little regard for the consequences. There is usually also a reduced need for sleep during manic phases. During periods of depression, the individual may experience crying and have a negative outlook on life and poor eye contact with others. The risk of suicide is high; over a period of 20 years, 6% of those with bipolar disorder died by suicide, while 30–40% engaged in self-harm. Other mental health issues, such as anxiety disorders and substance use disorders, are commonly associated with bipolar disorder. While the causes of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemolytic Anemia
Hemolytic anemia or haemolytic anaemia is a form of anemia due to hemolysis, the abnormal breakdown of red blood cells (RBCs), either in the blood vessels (intravascular hemolysis) or elsewhere in the human body (extravascular). This most commonly occurs within the spleen, but also can occur in the reticuloendothelial system or mechanically (prosthetic valve damage). Hemolytic anemia accounts for 5% of all existing anemias. It has numerous possible consequences, ranging from general symptoms to life-threatening systemic effects. The general classification of hemolytic anemia is either intrinsic or extrinsic. Treatment depends on the type and cause of the hemolytic anemia. Symptoms of hemolytic anemia are similar to other forms of anemia ( fatigue and shortness of breath), but in addition, the breakdown of red cells leads to jaundice and increases the risk of particular long-term complications, such as gallstones and pulmonary hypertension. Signs and symptoms Symptoms of hemolytic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SPR (gene)
Sepiapterin reductase is an enzyme that in humans is encoded by the ''SPR'' gene. Function Sepiapterin reductase (7,8-dihydrobiopterin:NADP+ oxidoreductase; EC 1.1.1.153) catalyzes the NADPH-dependent reduction of various carbonyl substances, including derivatives of pteridines, and belongs to a group of enzymes called aldo-keto reductases. SPR plays an important role in the biosynthesis of tetrahydrobiopterin. Reaction Sepiapterin reductase (SPR) catalyzes the chemical reaction L-erythro-7,8-dihydrobiopterin + NADP+ \rightleftharpoons sepiapterin + NADPH + H+ Thus, the two substrates of this enzyme are L-erythro-7,8-dihydrobiopterin and NADP+, whereas its three products are sepiapterin, NADPH, and a single hydrogen ion (H+). This enzyme belongs to the family of oxidoreductases, to be specific, those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is 7,8-dihydrobiopterin:NADP+ oxidoreductase. This enzyme p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements. A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |