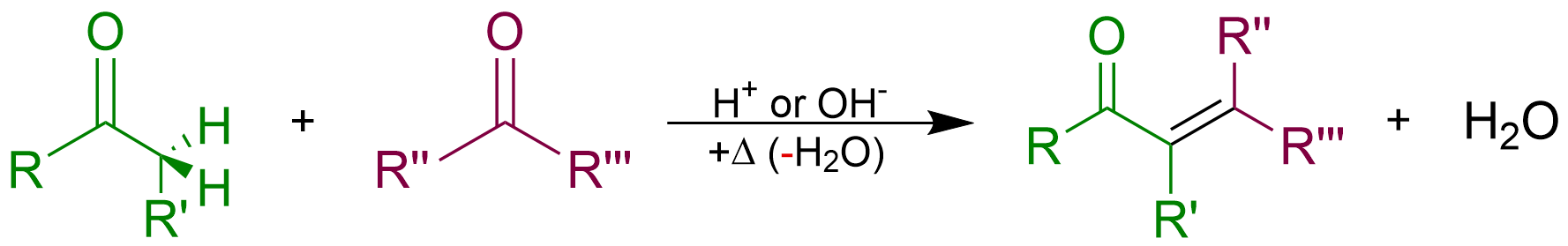|
Shogaol
Shogaols are pungent constituents of ginger similar in chemical structure to gingerol. The most common of the group is shogaol. Like zingerone, it is produced when ginger is dried or cooked. Moreover, shogaol (and gingerol) are converted to other constituents when heat is applied over time, which is why ginger loses its spiciness as it is cooked. The name ''shogaol'' is derived from the Japanese name for ginger (生姜、''shōga''). Shogaol is rated 160,000 SHU on the Scoville scale. When compared to other pungent compounds, shogaol is moderately more pungent than piperine, but less than capsaicin. Shogaols group Shogaol, shogaol, 0shogaol, and 2shogaol (all found in ginger) together constitute the group shogaols. There also exist in ginger cultivars methylated shogaols: methyl shogaol and methyl shogaol, respectively. Shogaols are artifacts formed during storage or through excess heat, probably created by a dehydration reaction of the gingerols. The ratio of shoga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gingerol
Gingerol ( gingerol) is a phenolic phytochemical compound found in fresh ginger that activates spice receptors on the tongue. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species. Cooking ginger transforms gingerol via a reverse aldol reaction into zingerone, which is less pungent and has a spicy-sweet aroma. When ginger is dried or mildly heated, gingerol undergoes a dehydration reaction forming shogaols, which are about twice as pungent as gingerol.NSF International ''Determination of Gingerols and Shogaols in Zingiber officinale rhizome and powdered extract by High-Performance Liquid Chromatography'' This explains why dried ginger is more pungent than fresh ginger. Ginger also contains gingerol, 0gingerol, and 2gingerol, collectively deemed g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ginger
Ginger (''Zingiber officinale'') is a flowering plant whose rhizome, ginger root or ginger, is widely used as a spice A spice is a seed, fruit, root, bark, or other plant substance primarily used for flavoring or coloring food. Spices are distinguished from herbs, which are the leaves, flowers, or stems of plants used for flavoring or as a garnish. Spice ... and a folk medicine. It is a herbaceous perennial plant, perennial which grows annual pseudostems (false stems made of the rolled bases of leaves) about one meter tall bearing narrow leaf blades. The inflorescences bear flowers having pale yellow petals with purple edges, and arise directly from the rhizome on separate shoots. Ginger is in the family (taxonomy), family Zingiberaceae, which also includes turmeric (''Curcuma longa''), cardamom (''Elettaria cardamomum''), and galangal. Ginger originated in Maritime Southeast Asia and was likely domesticated first by the Austronesian peoples. It was transported with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pungent
Pungency () refers to the taste of food commonly referred to as spiciness, hotness or heat, found in foods such as chili peppers. Highly pungent tastes may be experienced as unpleasant. The term piquancy () is sometimes applied to foods with a lower degree of pungency that are "agreeably stimulating to the palate". Examples of piquant food include mustard (condiment), mustard and curry. Terminology In colloquial speech, the term "pungency" can refer to any strong, sharp Olfaction, smell or Flavor (taste), flavor. However, in scientific speech, it refers specifically to the "hot" or "spicy" quality of chili peppers. It is the preferred term by scientists as it eliminates the potential ambiguity arising from use of "hot" and "spicy", which can also refer to temperature or the presence of spices, respectively. For instance, a pumpkin pie can be both hot (out of the oven) and spicy (due to the common inclusion of spices such as cinnamon, nutmeg, allspice, mace (spice), mace, and clo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pungent Flavors
Pungency () refers to the taste of food commonly referred to as spiciness, hotness or heat, found in foods such as chili peppers. Highly pungent tastes may be experienced as unpleasant. The term piquancy () is sometimes applied to foods with a lower degree of pungency that are "agreeably stimulating to the palate". Examples of piquant food include mustard and curry. Terminology In colloquial speech, the term "pungency" can refer to any strong, sharp smell or flavor. However, in scientific speech, it refers specifically to the "hot" or "spicy" quality of chili peppers. It is the preferred term by scientists as it eliminates the potential ambiguity arising from use of "hot" and "spicy", which can also refer to temperature or the presence of spices, respectively. For instance, a pumpkin pie can be both hot (out of the oven) and spicy (due to the common inclusion of spices such as cinnamon, nutmeg, allspice, mace, and cloves), but it is not ''pungent''. (A food critic may neverthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Journal Of Molecular Sciences
The ''International Journal of Molecular Sciences'' is a peer-reviewed open access scientific journal covering research in chemistry, molecular physics, and molecular biology. It is published by MDPI and was established in 2000. The editor-in-chief is Maurizio Battino (Marche Polytechnic University). Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 6.208. References External links * Biochemistry journals Open access journals MDPI academic journals English-language journals Monthly journals Publications established in 2000 {{Biochemistry-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofurane
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing ''n''-butane to crude maleic anhydride, followed by catalytic hydrogenation. A third major industrial route entails hydroformylation of allyl alcohol followed by hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexanal
Hexanal, also called hexanaldehyde or caproaldehyde is an alkyl aldehyde used in the flavor industry to produce fruity flavors. Its scent resembles freshly cut grass Poaceae () or Gramineae () is a large and nearly ubiquitous family of monocotyledonous flowering plants commonly known as grasses. It includes the cereal grasses, bamboos and the grasses of natural grassland and species cultivated in lawns a ..., like ''cis''-3-hexenal. It is potentially useful as a natural extract that prevents fruit spoilage. It occurs naturally, and contributes to a hay-like "off-note" flavor in green peas. The first synthesis of hexanal was published in 1907 by P. Bagard. / American Chemical Society References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanillin
Vanillin is an organic compound with the molecular formula . It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals. Vanillin and ethylvanillin are used by the food industry; ethylvanillin is more expensive, but has a stronger note. It differs from vanillin by having an ethoxy group (−O−CH2CH3) instead of a methoxy group (−O−CH3). Natural vanilla extract is a mixture of several hundred different compounds in addition to vanillin. Artificial vanilla flavoring is often a solution of pure vanillin, usually of synthetic origin. Because of the scarcity and expense of natural vanilla extract, synthetic preparation of its predominant component has long been of interest. The first commercial synthesis of vanillin began with the more readily available na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |