|
Sedoheptulose 1,7-bisphosphate
Sedoheptulose-bisphosphatase (also sedoheptulose-1,7-bisphosphatase or SBPase, EC number 3.1.3.37; systematic name sedoheptulose-1,7-bisphosphate 1-phosphohydrolase) is an enzyme that catalyzes the removal of a phosphate group from sedoheptulose 1,7-bisphosphate to produce sedoheptulose 7-phosphate. SBPase is an example of a phosphatase, or, more generally, a hydrolase. This enzyme participates in the Calvin cycle. Structure SBPase is a homodimeric protein, meaning that it is made up of two identical subunits. The size of this protein varies between species, but is about 92,000 Da (two 46,000 Da subunits) in cucumber plant leaves. The key functional domain controlling SBPase function involves a disulfide bond between two cysteine residues. These two cysteine residues, Cys52 and Cys57, appear to be located in a flexible loop between the two subunits of the homodimer, near the active site of the enzyme. Reduction of this regulatory disulfide bond by thioredoxin incites a con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroplast
A chloroplast () is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules ATP and NADPH while freeing oxygen from water in the cells. The ATP and NADPH is then used to make organic molecules from carbon dioxide in a process known as the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in unicellular algae, up to 100 in plants like ''Arabidopsis'' and wheat. A chloroplast is characterized by its two membranes and a high concentration of chlorophyll. Other plastid types, such as the leucoplast and the chromoplast, contain little chlorophyll and do not carry out photosynthesis. Chloroplasts are highly dynamic—they circulat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RuBisCO
Ribulose-1,5-bisphosphate carboxylase-oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme () involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is converted by plants and other photosynthesis, photosynthetic organisms to fuel, energy-rich molecules such as glucose. In chemical terms, it catalysis, catalyzes the carboxylation of ribulose-1,5-bisphosphate (also known as RuBP). It is probably the most abundant enzyme on Earth. Alternative carbon fixation pathways RuBisCO is important biology, biologically because it catalyzes the primary chemical reaction by which Total inorganic carbon, inorganic carbon enters the biosphere. While many autotrophic bacteria and archaea fix carbon via the reductive acetyl CoA Pathway, reductive acetyl CoA pathway, the 3-hydroxypropionate cycle, or the reverse Krebs cycle, these pathways are relatively small contributors to global carbon fixation compared t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructose 1,6-bisphosphatase
The enzyme fructose bisphosphatase (EC 3.1.3.11; systematic name D-fructose-1,6-bisphosphate 1-phosphohydrolase) catalyses the conversion of fructose-1,6-bisphosphate to fructose 6-phosphate in gluconeogenesis and the Calvin cycle, which are both anabolic pathways: :D-fructose 1,6-bisphosphate + H2O = D-fructose 6-phosphate + phosphate Phosphofructokinase (EC 2.7.1.11) catalyses the reverse conversion of fructose 6-phosphate to fructose-1,6-bisphosphate, but this is not just the reverse reaction, because the co-substrates are different (and so thermodynamic requirements are not violated). The two enzymes each catalyse the conversion in one direction only, and are regulated by metabolites such as fructose 2,6-bisphosphate so that high activity of one of them is accompanied by low activity of the other. More specifically, fructose 2,6-bisphosphate allosterically inhibits fructose 1,6-bisphosphatase, but activates phosphofructokinase-I. Fructose 1,6-bisphosphatase is involved in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
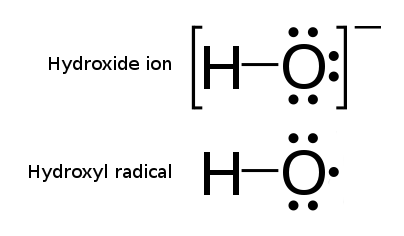
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound when exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen. The reduction of molecular oxygen () produces superoxide (), which is the precursor to most other reactive oxygen species: :O2 + e^- -> \ ^\bullet O2- Dismutation of superoxide produces hydrogen peroxide (): :2 H+ + \ ^\bullet O2^- + \ ^\bullet O2^- -> H2O2 + O2 Hydrogen peroxide in turn may be partially reduced, thus forming hydroxide ions and hydroxyl radicals (), or fully reduced to water: :H2O2 + e^- -> HO^- + \ ^\bullet OH :2 H+ + 2 e- + H2O2 -> 2 H2O In a biological context, ROS are byproducts of the normal metabolism of oxygen. ROS have roles in cell signaling and homeostasis. ROS are intrinsic to cellular functioning, and are present at low and stationary levels in normal cells. In plants, ROS are involved in metabolic processes related to photoprotection and toleran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains. Organic chemistry Several industrially useful organic chemicals are prepared by carbonylations, which can be highly selective reactions. Carbonylations produce organic carbonyls, i.e., compounds that contain the C=O functional group such as aldehydes, carboxylic acids and esters. Carbonylations are the basis of many types of reactions, including hydroformylation and Reppe reactions. These reactions require metal catalysts, which bind and activate the CO. These processes involve transition metal acyl complexes as intermediates. Much of this theme was developed by Walter Reppe. Hydroformylation Hydroformylation entails the addition of both carbon monoxide and hydrogen to unsaturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
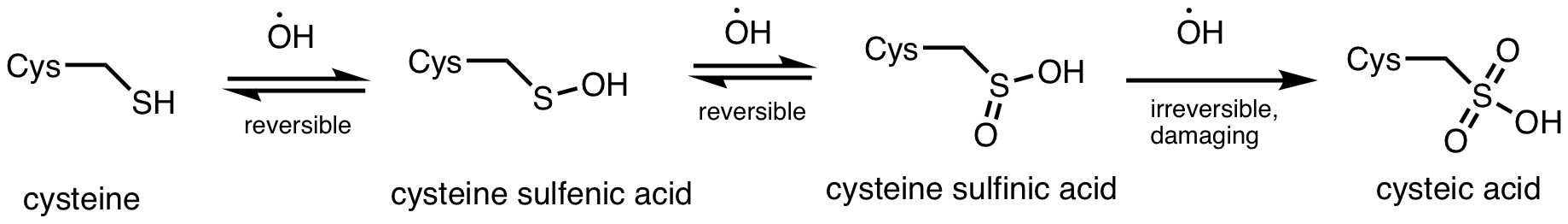
Cysteine Carbonylation
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SBPase Regulation By Ferredoxin-thioredoxin System
Sedoheptulose-bisphosphatase (also sedoheptulose-1,7-bisphosphatase or SBPase, EC number 3.1.3.37; systematic name sedoheptulose-1,7-bisphosphate 1-phosphohydrolase) is an enzyme that catalyzes the removal of a phosphate group from sedoheptulose 1,7-bisphosphate to produce sedoheptulose 7-phosphate. SBPase is an example of a phosphatase, or, more generally, a hydrolase. This enzyme participates in the Calvin cycle. Structure SBPase is a homodimeric protein, meaning that it is made up of two identical subunits. The size of this protein varies between species, but is about 92,000 Da (two 46,000 Da subunits) in cucumber plant leaves. The key functional domain controlling SBPase function involves a disulfide bond between two cysteine residues. These two cysteine residues, Cys52 and Cys57, appear to be located in a flexible loop between the two subunits of the homodimer, near the active site of the enzyme. Reduction of this regulatory disulfide bond by thioredoxin incites a conforma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
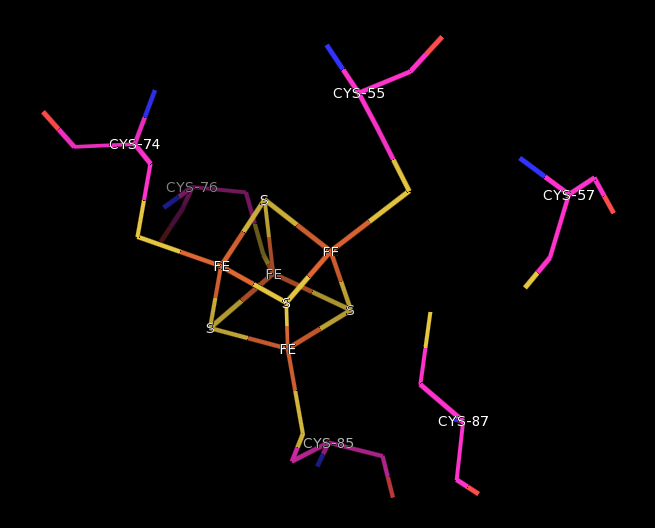
Ferredoxin-thioredoxin Reductase
Ferredoxin-thioredoxin reductase , systematic name ''ferredoxin:thioredoxin disulfide oxidoreductase,'' is a [4Fe-4S] protein that plays an important role in the ferredoxin/thioredoxin regulatory chain. It catalyzes the following reaction: ::: 2 reduced ferredoxin + thioredoxin disulfide \rightleftharpoons 2 oxidized ferredoxin + thioredoxin thiols + 2 H+ Ferredoxin-Thioredoxin reductase (FTR) converts an electron signal (photoreduced ferredoxin) to a thiol signal (reduced thioredoxin), regulating enzymes by Redox, reduction of specific disulfide groups. It catalysis, catalyses the light-dependent activation of several photosynthesis enzymes and constitutes the first historical example of a thiol/disulfide exchange cascade for enzyme regulation. It is a heterodimer of subunit alpha and subunit beta. Subunit alpha is the variable subunit, and beta is the catalytic chain. The secondary structure, structure of the beta subunit has been determined and found to fold around the FeS cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SBPase Reaction Scheme
Sedoheptulose-bisphosphatase (also sedoheptulose-1,7-bisphosphatase or SBPase, EC number 3.1.3.37; systematic name sedoheptulose-1,7-bisphosphate 1-phosphohydrolase) is an enzyme that catalyzes the removal of a phosphate group from sedoheptulose 1,7-bisphosphate to produce sedoheptulose 7-phosphate. SBPase is an example of a phosphatase, or, more generally, a hydrolase. This enzyme participates in the Calvin cycle. Structure SBPase is a homodimeric protein, meaning that it is made up of two identical subunits. The size of this protein varies between species, but is about 92,000 Atomic mass unit, Da (two 46,000 Da subunits) in cucumber plant leaves. The key functional domain controlling SBPase function involves a disulfide bond between two cysteine residues. These two cysteine residues, Cys52 and Cys57, appear to be located in a flexible loop between the two subunits of the homodimer, near the active site of the enzyme. Reduction of this regulatory disulfide bond by thioredoxin i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |